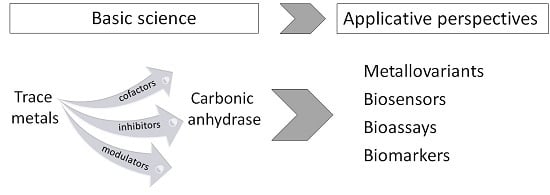
The Complex Relationship between Metals and Carbonic Anhydrase: New Insights and Perspectives
Abstract
:1. Introduction
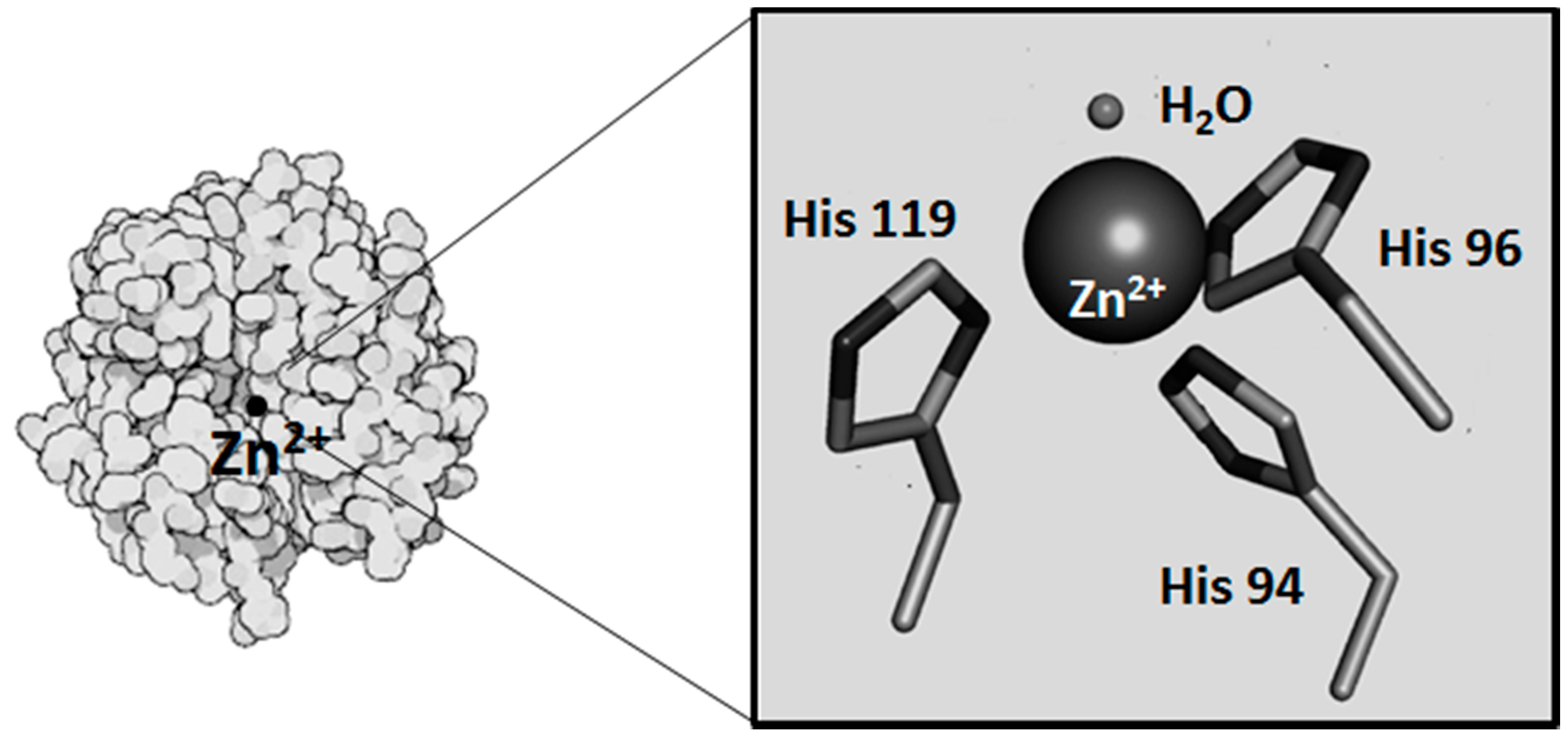
2. Metals and CA Catalytic Site
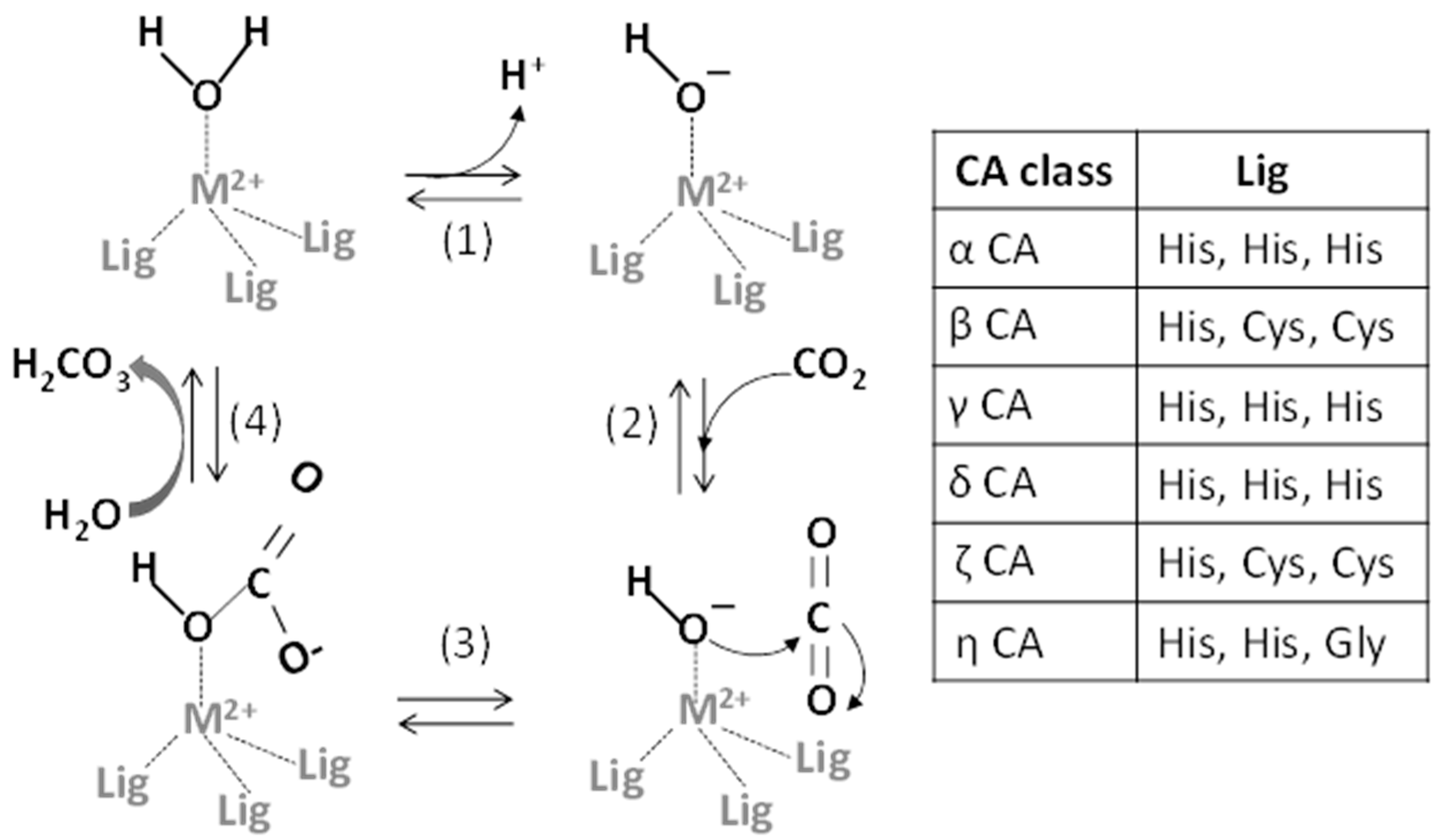
| CA Families | Metals as Physiologically-Relevant CA Cofactors | Ref. |
|---|---|---|
| α-CA | Zn2+ | [21] |
| β-CA | Zn2+ | [7] |
| γ-CA | Fe2+; Zn2+ | [9,22] |
| δ-CA | Zn2+ | [10] |
| ζ-CA | Cd2+; Zn2+ | [12] |
| η-CA | Zn2+ | [2] |
2.1. Metals as Physiologically-Relevant Cofactors of CA
2.2. Metals as Displacers of the Native Metal Cofactor
2.3. Metal Binding to Other Sites in the CA Protein
3. Metals and CA Activity Inhibition
| Species | Tissue | Cd2+ (mM) | Cu2+ | Hg2+ | Zn2+ | Co2+ | Pb2+ | Ag+ | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Callinectes sapidus | gills | Ki 0.1 × 10−3 | Ki 3.6 × 10−3 | Ki 2–6 × 10−3 | Ki 0.05 × 10-3 | [51] | |||
| Carcinus maenas | gills | Ki 0.6–2.5 | Ki 0.6–2.5 | Ki 0.6–2.5 | [51] | ||||
| Anguilla anguilla | gills | IC50 9.9 × 10−3 | [46] | ||||||
| Anguilla anguilla | intestine | IC50 36.4 × 10−3 | [46] | ||||||
| Sparus aurata | liver | Ki 17.7 (non-competitive) | Ki 36.2 (non-competitive) | Ki 0.02 (non-competitive) | [48] | ||||
| Ictalurus punctatus | erythrocyte | IC50 0.9 | IC50 0.065 | IC50 0.7 | IC50 0.035 | [44] | |||
| Oncorhynchus mykiss | brain | Ki 94.2 × 10−3 | Ki 27.6 × 10−3 (non-competitive) | Ki 1.20 | Ki 0.035 | [49] | |||
| Acipenser gueldenstaedti | erythrocyte | IC50 5.2 | IC50 2.8 | IC50 1.7 | [53] | ||||
| Dicentrarchus labrax | liver | Ki 0.76 | Ki 0.72 (competitive) | Ki 0.53 (competitive) | Ki 0.24 (competitive) | [47] | |||
| Gallus | erythrocyte | Ki 2.78 (competitive) | Ki 1.26 (competitive) | Ki 0.97 (competitive) | [55] | ||||
| Ovis aries | kidney | Ki 1.04 (competitive) | Ki 4.70 (competitive) | Ki 0.96 (competitive) | [54] | ||||
| Homo sapiens | Erythrocyte, CA I | Ki 3.22 | Ki 3.22 (uncompetitive) | Ki 1.45 (competitive) | Ki 1 (non-competitive) | [52] | |||
| Homo sapiens | Erythrocyte, CA II | Ki 0.312 | Ki 0.312 (uncompetitive) | Ki 1.7 (non-competitive) | Ki 0.056 (competitive) | [52] |
4. Metals and CA Protein Expression
5. CA and Trace Metals: Applicative Insights and Perspectives
5.1. CA Metallovariants
5.2. CA-Based Biosensors for Metal Ions
5.3. CA-Based Ecotoxicological Biomarkers and Bioassays for Metal Pollution Assessment
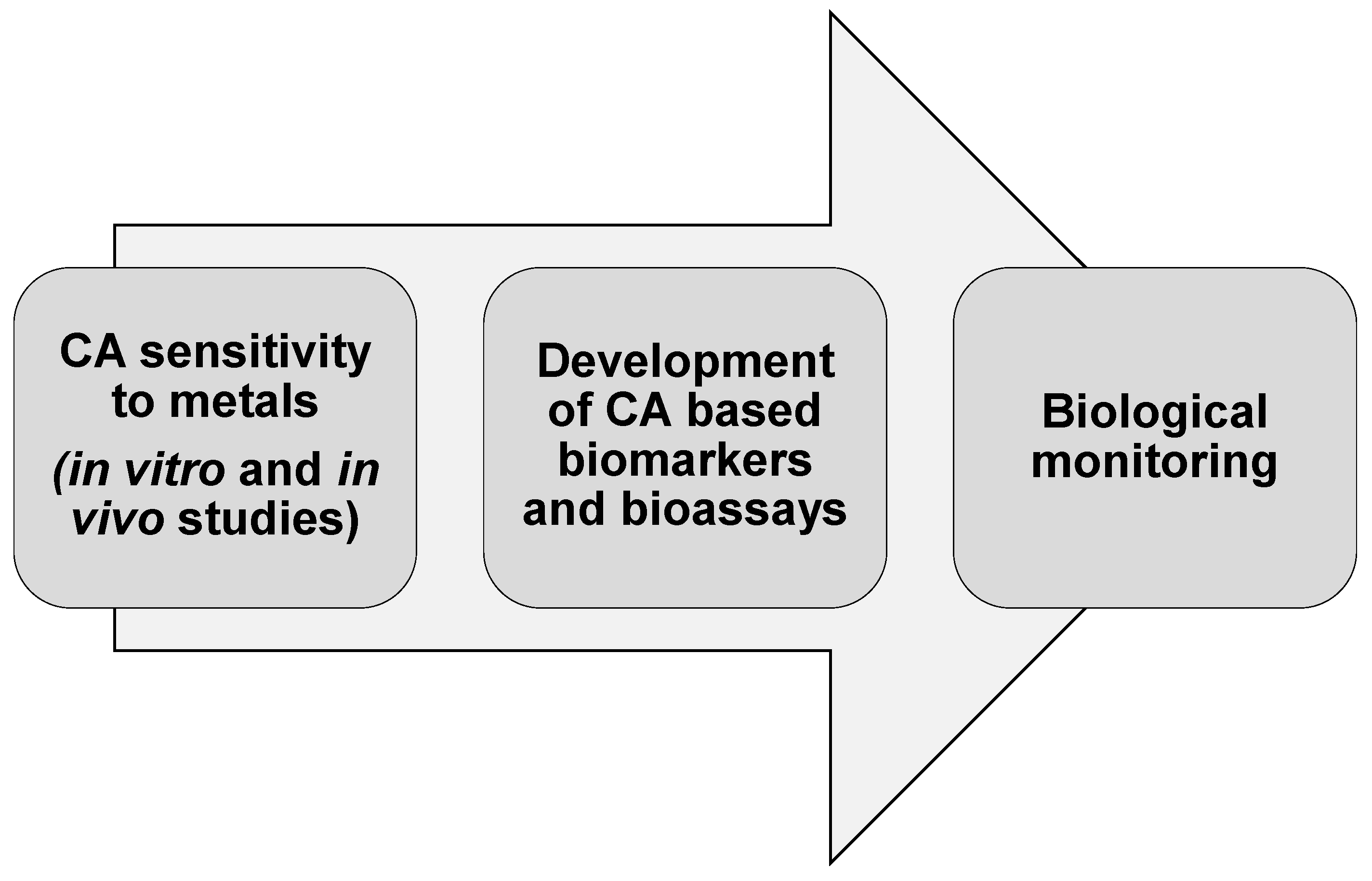
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Supuran, C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; Fisher, G.M.; Andrews, K.T.; Poulsen, S.A.; Capasso, C.; Supuran, C.T. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum—The η-carbonic anhydrases. Bioorg. Med. Chem. Lett. 2014, 24, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, B.N.; Ignatova, L.K.; Romanova, A.K. Diversity in forms and functions of carbonic anhydrase in terrestrial higher plants. Russ. J. Plant Physiol. 2007, 54, 143–162. [Google Scholar] [CrossRef]
- Cannon, G.C.; Heinhorst, S.; Kerfeld, C.A. Carboxysomal carbonic anhydrases: Structure and role in microbial CO2 fixation. BBA Proteins Proteom. 2010, 1804, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumors as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Kimber, M.S.; Pai, E.F. The active site architecture of Pisum sativum β-carbonic anhydrase is a mirror image of that of α-carbonic anhydrases. EMBO J. 2000, 19, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Ferry, J.G. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef] [PubMed]
- Alber, B.E.; Ferry, J.G. A carbonic anhydrase from the archeon Methanosarcina thermophile. Proc. Natl. Acad. Sci. USA 1994, 91, 6909–6913. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Lane, T.W.; Morel, F.M.M. Carbonic anhydrase in the marine diatom Thalassiosira weissflogii (Bacillariophyceae). J. Phycol. 1997, 33, 845–850. [Google Scholar] [CrossRef]
- Tripp, B.C.; Smith, K.S.; Ferry, J.G. Carbonic anhydrase: New insights for an ancient enzyme. J. Biol. Chem. 2001, 276, 48615–48618. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Supuran, C.T.; Morel, F.M.M. Cadmium-Carbonic Anhydrase. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Supuran, C.T.; Scozzafava, A.; Casini, A. Carbonic anhydrase inhibitors. Med. Res. Rev. 2003, 23, 146–189. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Scozzafava, A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg. Med. Chem. Lett. 2007, 15, 4336–4350. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Scozzafava, S.; Parkkila, L.; Puccetti, G.; de Simone, G.; Supuran, C.T. Investigations of the esterase, phosphatase, and sulfatase activities of the cytosolic mammalian carbonic anhydrase isoforms I, II, and XIII with 4-nitrophenyl esters as substrates. Bioorg. Med. Chem. Lett. 2008, 18, 2267–2271. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; di Fiore, A.; Capasso, C.; Supuran, C.T. The zinc coordination pattern in the eta-carbonic anhydrase from Plasmodium falciparum is different from all other carbonic anhydrase genetic families. Bioorg. Med. Chem. Lett. 2015, 25, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 7th ed.; W.H. Freeman: New York, NY, USA, 2012. [Google Scholar]
- Williams, R.J.P. The biochemistry of zinc. Polyhedron 1987, 6, 61–69. [Google Scholar] [CrossRef]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Vahrenkamp, H. Why does nature use zinc—A personal view. Dalton Trans. 2007, 42, 4751–4759. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- MacAuley, S.R.; Zimmerman, S.A.; Apolinario, E.E.; Evilia, C.; Hou, Y.; Ferry, J.G.; Sowers, K.R. The archetype γ-class carbonic anhydrase (Cam) contains iron when synthesized in vivo. Biochemistry 2009. [Google Scholar] [CrossRef] [PubMed]
- Tripp, B.C.; Bell, C.B.; Cruz, F.; Krebs, C.; Ferry, J.G. A role for iron in an ancient carbonic anhydrase. J. Biol. Chem. 2004, 279, 21677–21677. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.G. The gamma class of carbonic anhydrases. Biochim. Biophys. Acta 2010, 1804, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, X.; Li, P.; Huang, H. Impact of Zn, Cu, and Fe on the Activity of Carbonic Anhydrase of Erythrocytes in Ducks. Biol. Trace Elem. Res. 2007, 118, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.W.; Morel, F.M.M. A biological function for cadmium in marine diatoms. Proc. Natl. Acad. Sci. USA 2000, 97, 4627–4631. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.W.; Saito, M.A.; George, G.N.; Pickering, I.J.; Prince, R.C.; Morel, F.M.M. Biochemistry: A cadmium enzyme from a marine diatom. Nature 2005, 435. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; McGinn, P.J.; Morel, F.M.M. Expression of cadmium carbonic anhydrase of diatoms in seawater. Aquat. Microbial. Ecol. 2008, 51, 183–193. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Lionetto, M.G.; Vilella, S.; Trischitta, F.; Cappello, M.S.; Giordano, M.E.; Schettino, T. Effects of CdCl2 on electrophysiological parameters in the intestine of the teleost fish, Anguilla anguilla. Aquat. Toxicol. 1998, 41, 251–264. [Google Scholar]
- Yee, D.; Morel, F.M.M. In vivo substitution of zinc by cobalt in carbonic anhydrase of a marine diatom. Limnol. Oceanogr. 1996, 41, 573–577. [Google Scholar] [CrossRef]
- Mahon, B.P.; Pinar, M.A.; McKenna, R. Targeting Carbonic Anhydrase IX Activity and Expression. Molecules 2015, 20, 2323–2348. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Goodsell, D. Carbonic anhydrase. Available online: http://pdb101.rcsb.org/motm/49 (accessed on 12 January 2016).
- Håkansson, K.; Wehnert, A.; Liljas, A. X-ray analysis of metal-substituted human carbonic anhydrase II derivatives. Acta Crystallogr. D 1994, 50, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kogut, K.A.; Rowlett, R.S. A comparison of the mechanisms of CO2 hydration by native and Co2+-substituted carbonic anhydrase II. J. Biol. Chem. 1987, 262, 16417–16424. [Google Scholar] [PubMed]
- Marino, T.; Russo, N.; Toscano, M. A Comparative Study of the Catalytic Mechanisms of the Zinc and Cadmium Containing Carbonic Anhydrase. J. Am. Chem. Soc. 2005, 127, 4242–4253. [Google Scholar] [CrossRef] [PubMed]
- Led, J.J.; Neesgaard, E. Paramagnetic Carbon-13 NMR Studies of the Kinetics and Mechanism of the HCO3/CO2 Exchange Catalyzed by Manganese(II) Human Carbonic Anhydrase I. Biochemistry 1987, 26, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Sven, L. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar]
- Hoffmann, K.M.; Samardzic, D.; van den Heever, K.; Rowlett, R.S. Co(II)-substituted Haemophilus influenzae b-carbonic anhydrase: Spectral evidence for allosteric regulation by pH and bicarbonate ion. Arch. Biochem. Biophys. 2011, 511, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.A.; Kylsten, P.M.; Jones, A.T.; Liljas, A. Crystallographic studies of inhibitor binding sites in human carbonic anhydrase II; A penta coordinated binding of the SCN- ion to the zinc at high pH. Proteins 1988, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Hogeback, J.; Schwarzer, M.; Wehe, C.A.; Sperlingab, M.; Karst, U. Investigating the adduct formation of organic mercury species with carbonic anhydrase and hemoglobin from human red blood cell hemolysate by means of LC/ESI-TOF-MS and LC/ICP-MS. Metallomics 2015. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Wynns, G.C.; Silverman, D.N. Inhibition by cupric ions of 18O exchange catalyzed by human carbonic anhydrase II. Relation to the interaction between carbonic anhydrase and hemoglobin. J. Biol. Chem. 1981, 256, 9466–9470. [Google Scholar] [PubMed]
- Song, H.; Weitz, A.C.; Hendrich, M.P.; Lewis, E.A.; Emerson, J.P. Building reactive copper centers in human carbonic anhydrase II. J. Biol. Inorg. Chem. 2013, 18, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Nettles, W.L.; Song, H.; Farquhar, E.R.; Fitzkee, N.C.; Emerson, J.P. Characterization of the copper(II) binding sites in human carbonic anhydrase II. Inorg. Chem. 2015, 54, 5671–5680. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.M.; Tucker, J.H. Effects of selected water toxicants on the in vitro activity of fish carbonic anhydrase. Chem. Biol. Interact. 1976, 13, 181–192. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Maffia, M.; Cappello, M.S.; Giordano, M.E.; Storelli, C.; Schettino, T. Effect of cadmium on carbonic anhydrase and Na+-K+-ATPase in eel, Anguilla anguilla, intestine and gills. Comp. Biochem. Physiol. A 1998, 120, 89–91. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Giordano, M.E.; Vilella, S.; Schettino, T. Inhibition of eel enzymatic activities by cadmium. Aquat. Toxicol. 2000, 48, 561–571. [Google Scholar] [CrossRef]
- Ceyhun, S.B.; Şentürk, M.; Yerlikaya, E.; Erdoğan, O.; Küfrevioğlu, Ö.I.; Ekinci, D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European Seabass) liver and toxicological effects of metals on enzyme activity. Environ. Toxicol. Pharmacol. 2011, 32, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.D.; Söyüt, H.; Beydemir, S. Carbonic anhydrase activity from the gilthead sea bream (Sparus aurata) liver: The toxicological effects of heavy metals. Environ. Toxicol. Pharmacol. 2013, 36, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Soyut, H.; Beydemir, Ş.; Hisar, O. Effects of some metals on carbonic anhydrase from brains of rainbow trout. Biol. Trace Elem. Res. 2008, 123, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.M.; Monserrat, J.M.; Casthilo, P.; Rodriguez, E.M. Inhibitory effects of cadmium on carbonic anhydrase activity and ionic regulation of the estuarine crab, Chasmagnathus granulata (Decapoda, Grapsidae). Comp. Biochem. Physiol. C 1999, 122, 121–129. [Google Scholar] [CrossRef]
- Skaggs, H.S.; Henry, R.P. Inhibition of carbonic anhydrase in the gills of two euryhaline crabs, Callinectes sapidus and Carcinus maenas, by heavy metals. Comp. Biochem. Physiol. C 2002, 133, 605–612. [Google Scholar] [CrossRef]
- Ekinci, D.; Beydemir, Ş.; Küfrevioğlu, Ö.İ. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2007, 22, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Kolayli, S.; Karahalil, F.; Sahin, H.; Dicer, B.; Supuran, C.T. Characterization and inhibition studies of an α-carbonic anhydrase from the endangered sturgeon species Acipenser gueldenstaedti. J. Enzym. Inhib. Med. Chem. 2011, 26, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Demirdağ, R.; Comakli, V.; Kuzu, M.; Yerlikaya, E.; Şentürk, M. Purification and characterization of carbonic anhydrase from Ağrı Balık Lake Trout Gill (Salmo trutta labrax) and effects of sulfonamides on enzyme activity. J. Biochem. Mol. Toxicol. 2013, 29, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Mercan, L.; Ekinci, D.; Supuran, C.T. Characterization of carbonic anhydrase from Turkish native “Gerze” chicken and influences of metal ions on enzyme activity. J. Enzym. Inhib. Med. Chem. 2014, 29, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Jebali, J.; Chouba, L.; Bannia, M.; Boussetta, H. Comparative study of the bioaccumulation and elimination of trace metals (Cd, Pb, Zn, Mn and Fe) in the digestive gland, gills and muscle of bivalve Pinna nobilis during a field transplant experiment. J. Trace Elem. Med. Biol. 2014, 28, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Caricato, R.; Erroi, E.; Giordano, M.E.; Schettino, T. Potential application of carbonic anhydrase activity in bioassay and biomarker studies. Chem. Ecol. 2006, 22, S119–S125. [Google Scholar] [CrossRef]
- Soto, M.; Ireland, M.P.; Marigómez, I. Changes in mussel biometry on exposure to metals: Implications in estimation of metal bioavailability in “Mussel-Watch” programmes. Sci. Total Environ. 2000, 247, 175–187. [Google Scholar] [CrossRef]
- Brock, J.R.; Bielmyer, G.K. Metal accumulation and sublethal effects in the sea anemone, Aiptasia pallida, after waterborne exposure to metal mixtures. Comp. Biochem. Physiol. C 2013, 158, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Santini, O.; Chahbane, N.; Vasseur, P.; Frank, H. Effects of low-level copper exposure on Ca2+-ATPase and carbonic anhydrase in the freshwater bivalve Anodonta anatine. Toxicol. Environ. Chem. 2011, 93, 1826–1837. [Google Scholar] [CrossRef]
- Bielmyer, G.K.; Grosell, M.; Bhagooli, R.; Baker, A.C.; Langdon, C.; Gillette, P.; Capo, T.R. Differential effects of copper on three species of scleractinian corals and their algal symbionts (Symbiodinium spp.). Aquat. Toxicol. 2010, 97, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.L.; Guzman, H.M. Bioindication potential of carbonic anhydrase activity in anemones and corals. Mar. Pollut. Bull. 2001, 42, 742–744. [Google Scholar] [CrossRef]
- Khan, N.A.; Singh, S.; Anjum, N.A.; Nazar, R. Cadmium effects on carbonic anhydrase, photosynthesis, dry mass and antioxidative enzymes in wheat (Triticum aestivum) under lowand sufficient zinc. J. Plant Interact. 2008, 3, 31–37. [Google Scholar] [CrossRef]
- Henkin, R.; Martin, B.M.; Agarwal, R. Efficacy of exogenous oral zinc in treatment of patients with carbonic anhydrase VI deficiency. Am. J. Med. Sci. 1999, 318, 392–405. [Google Scholar] [CrossRef]
- Goto, T.; Shirakawa, H.; Furukawa, Y.; Komai, M. Decreased expression of carbonic anhydrase isozyme II, rather than of isozyme VI, in submandibular glands in long term zinc-deficient rats. Br. J. Nutr. 2008, 99, 248–253. [Google Scholar] [CrossRef] [PubMed]
- De Polo, A.; Margiotta-Casaluci, L.; Lockyer1, A.E.; Scrimshaw, M.D. A new role for carbonic anhydrase 2 in the response of fish to copper and osmotic stress: Implications for multi-stressor studies. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Caricato, R.; Lionetto, M.G.; Dondero, F.; Viarengo, A.; Schettino, T. Carbonic anhydrase activity in Mytilus galloprovincialis digestive gland: Sensitivity to heavy metal exposure. Comp. Biochem. Physiol. C 2010, 152, 241–247. [Google Scholar]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Erroi, E.; Schettino, T. Carbonic anydrase and chemical pollutants: New applied perspectives. In Proceedings of the 65th Annual Meeting of the Italian Physiological Society, Anacapri, Italy, 28–30 September 2014.
- Köhler, A. Lysosomal perturbations in fish liver as indicators for toxic effects of environmental pollution. Comp. Biochem. Physiol. 1991, 100, 123–127. [Google Scholar] [CrossRef]
- Moore, M.N.; Simpson, M.G. Molecular and cellular pathology in environmental impact assessment. Aquat. Toxicol. 1992, 22, 313–322. [Google Scholar] [CrossRef]
- Jing, Q.; Okrasa, K.; Kazlauskas, R.J. Manganese-Substituted α-Carbonic Anhydrase as an Enantioselective Peroxidase. Top. Organomet. Chem. 2009, 25, 45–61. [Google Scholar]
- Okrasa, K.; Kazlauskas, R.J. Manganese-Substituted Carbonic Anhydrase as a New Peroxidase. Chem. Eur. J. 2006, 12, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- De Mora, S.; Fowler, S.W.; Wyse, E.; Azemard, S. Distribution of heavy metals in marine bivalves, fish and coastal sediments in the Gulf and Gulf of Oman. Mar. Pollut. Bull. 2004, 49, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.H.; Thompson, R.B.; Maliwal, B.P.; Fones, G.R.; Moffett, J.W.; Fierke, C.A. Real-time determination of picomolar free Cu(II) in seawater using a fluorescence-based fiber optic biosensor. Anal. Chem. 2003, 75, 6807–6812. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.; Hurst, T.K.; Westerberg, N.; Stoddard, A.; Fierke, C.A.; Frederickson, C.J.; Thompson, R.B. Determination of zinc using carbonic anhydrase-based fluorescence biosensors. Methods Enzymol. 2008, 450, 287–309. [Google Scholar] [PubMed]
- Fierke, C.A.; Thompson, R.B. Fluorescence-based biosensing of zinc using carbonic anhydrase. Biometals 2001, 14, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.J.; Frederickson, C.J.; Giblin, L.J.; Weiss, J.H.; Medvedeva, Y.; Bentley, P.A. Sensitive and selective detection of zinc ions in neuronal vesicles using PYDPY1, a simple turn-on dipyrrin. Chem. Commun. 2011, 47, 7107–7109. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.A.; Fierke, C.A. Probing determinants of the metal ion selectivity in carbonic anhydrase using mutagenesis. Biochemistry 2004, 43, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.B.; Bozym, R.A.; Cramer, M.L.; Stoddard, A.K.; Westerberg, N.M.; Fierke, C.A. Chapter 6: Carbonic anhydrase-based biosensing of metal ions: Issue and future prospects. In Fluorescence Sensors and Biosensors; Thompson, R.B., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Costa, C.R.; Olivi, P.; Botta, C.M.R.; Espindola, E.L.G. Toxicity in aquatic environments: Discussion and evaluation methods. Quim Nova 2008, 31, 1820–1830. [Google Scholar] [CrossRef]
- Manzo, S.; de Nicola, F.; de Luca Picione, F.; Maisto, G.; Alfani, A. Assessment of the effects of soil PAH accumulation by a battery of ecotoxicological tests. Chemosphere 2008, 71, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Forbes, V.E.; Palmqvist, A.; Bach, L. The use and misuse of biomarkers in ecotoxicology. Environ. Toxicol. Chem. 2006, 25, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Schettino, T.; Caricato, R.; Calisi, A.; Giordano, M.E.; Lionetto, M.G. Biomarker approach in marine monitoring and assessment: New insights and perspectives. Open Environ. Sci. 2012, 6, 20–27. [Google Scholar] [CrossRef]
- Martίnez-Gόmez, C.; Vethaak, A.D.; Hylland, K.; Burgeot, T.; Khöler, A.; Lyons, B.P.; Thain, J.; Gubbins, M.J.; Davies, I.M. A guide to toxicity assessment and monitoring effects at lower levels of biological organization following marine oil spills in European waters. J. Mar. Sci. 2010, 67, 1105–1118. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Erroi, E.; Schettino, T. Carbonic anhydrase as pollution biomarker: An ancient enzyme with a new use. Int. J. Environ. Res. Public Health 2012, 9, 3965–3977. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Brito, I.; Freire, C.A.; Yamamoto, F.Y.; de Assis, H.C.S.; Souza-Bastos, L.R.; Cestari, M.M.; de Castilhos Ghisi, N.; Prodocimo, V.; Filipak Neto, F.; de Oliveira Ribeiro, C.A. Monitoring water quality in reservoirs for human supply through multi-biomarker evaluation in tropical fish. J. Environ. Monit. 2012, 14, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Caricato, R.; Lionetto, M.G.; Schettino, T. Studio di biomarkers in mitili (Mytilus galloprovincialis) traslocati in Mar Piccolo e in Mar Grande di Taranto. Biol. Mar. Medit. 2009, 16, 136–147. [Google Scholar]
- Azevedo-Linhares, M.; Freire, C.A. Evaluation of impacted Brazilian estuaries using the native oyster Crassostrea rhizophorae: Branchial carbonic anhydrase as a biomarker. Ecotoxicol. Environ. Saf. 2015, 122, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Caricato, R.; Erroi, E.; Giordano, M.E.; Schettino, T. Carbonic anhydrase based environmental bioassay. Int. J. Environ. Anal. Chem. 2005, 85, 895–903. [Google Scholar] [CrossRef]
- Schettino, T.; Lionetto, M.G.; Erroi, E. Method for enzymatic assessment of the toxicity of environmental aqueous matrices. Patent WO 2009/135537, 2 March 2012. [Google Scholar]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Erroi, E.; Schettino, T. Biomonitoring of water and soil quality: A case study of ecotoxicological methodology application to the assessment of reclaimed agroindustrial wastewaters used for irrigation. Rend. Fis. Acc. Lincei 2015. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Schettino, T. The Complex Relationship between Metals and Carbonic Anhydrase: New Insights and Perspectives. Int. J. Mol. Sci. 2016, 17, 127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010127
Lionetto MG, Caricato R, Giordano ME, Schettino T. The Complex Relationship between Metals and Carbonic Anhydrase: New Insights and Perspectives. International Journal of Molecular Sciences. 2016; 17(1):127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010127
Chicago/Turabian StyleLionetto, Maria Giulia, Roberto Caricato, Maria Elena Giordano, and Trifone Schettino. 2016. "The Complex Relationship between Metals and Carbonic Anhydrase: New Insights and Perspectives" International Journal of Molecular Sciences 17, no. 1: 127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010127