The Sequence Characteristics and Expression Models Reveal Superoxide Dismutase Involved in Cold Response and Fruiting Body Development in Volvariella volvacea
Abstract
:1. Introduction
2. Results
2.1. Annotation and Sequence Accuracy Verification of SOD Encoding Genes
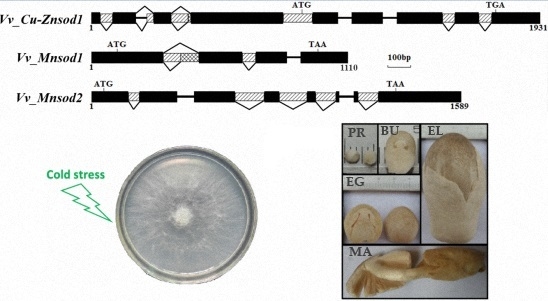
2.2. Genetic Structure and Alternative Splicing Analysis

2.3. Alignment and Phylogenetic Analysis of Amino Acid Sequences


2.4. Differential Expression of Sod Genes under Short-Term Cold Stress

2.5. Expression Patterns of Sod Genes during the Fruiting-body Development Stages of V. volvacea


3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Genome and Transcriptome Sequencing Data
4.3. Identification of the SOD Proteins of V. volvacea
4.4. Acquisition, Accuracy Verification and Structural Analysis of Full-Length Gene Sequences
4.5. Bioinformatics Analysis
4.6. Cold Stress Treatment
4.7. Mycelia and Fruiting-Body Growth and Sample Collection
4.8. DNA Extraction, PCR Amplification, and Sequence Determination
| Gene | Primer Sequence(5’-3’) | Annealing Temperature for PCR |
|---|---|---|
| Vv_Cu-Znsod1 | GGTTGCCGTCCTGTCCTTCTT | 58.0 °C |
| CGCCTGTAGTATTCTTGCCCTCAT | ||
| Vv_Mnsod1 | GACCAACGACCTTGATCCATC | 54.6 °C |
| GGACAACCAACGCCTGATG | ||
| Vv_Mnsod2 | GGTGAGAGTTGGAAGCGGTA | 54.9 °C |
| GGCACTGGACAGAGCGTAT | ||
| GME5781 | TTCCTTTACGCTGCCAACT | 55.4 °C |
| GTACGGCTGTGAATATGTCGA |
4.9. RNA Extraction and Reverse Transcription
4.10. RT-qPCR Assay of Gene Expression
| Gene | Forward Sequence (5’-3’) | Reverse Sequence (5’-3’) |
|---|---|---|
| Vv_Cu-Znsod1 | ACATTCGTCGCTGCTCTCCT | GTGAAGTTGACTGTGCCTGTGA |
| Vv_Mnsod1 | CGTTACCACCGCCAACCAAGA | TTAGGAGAGACCCAACCCTTAAAGC |
| Vv_Mnsod2 | TGCTCGCCATCGCCAGAACT | GGTGGTGCTTGGTGTGGTGAAG |
| gapdh | CATCTTCCACTGGTGCGGCTAAG | GGCTTCTCAAGGCGAACGACAA |
4.11. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, W.; Shi, Z.; Zhang, G. Research progress of superoxide dismutase. J. Mod. Agric. 2013, 2, 1–12. (In Chinese) [Google Scholar]
- Miller, A.F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.V.; Bannister, W.H.; Rotilio, G. Aspects of the structure, function, and applications of superoxide dismutase. Crit. Rev. Biochem. Mol. Biol. 1987, 22, 111–180. [Google Scholar] [CrossRef]
- Bafana, A.; Dutt, S.; Kumar, A.; Kumar, S.; Ahuja, P.S. The basic and applied aspects of superoxide dismutase. J. Mol. Catal. B Enzym. 2011, 68, 129–138. [Google Scholar] [CrossRef]
- Dong, L.; He, Y.; Wang, Y.; Dong, Z. Research progress on application of superoxide dismutase (SOD). J. Agric. Sci. Technol. 2013, 15, 53–58. (In Chinese) [Google Scholar]
- Oberley, L.W.; Oberley, T.D.; Buettner, G.R. Cell differentation, aging and cancer: The possible roles of superoxide and superoxide dismutases. Med. Hypotheses 1980, 6, 249–268. [Google Scholar] [CrossRef]
- Clair, D.K.S.; Oberley, T.D.; Muse, K.E.; Clair, W.H.S. Expression of manganese superoxide dismutase promotes cellular differentiation. Free Radic. Biol. Med. 1994, 16, 275–282. [Google Scholar]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Landis, G.N.; Tower, J. Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev. 2005, 126, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Rhee, J.S.; Park, G.S.; Lee, J.; Lee, Y.M.; Lee, J.S. Cu/Zn-and Mn-superoxide dismutase (SOD) from the copepod Tigriopus japonicus: Molecular cloning and expression in response to environmental pollutants. Chemosphere 2011, 84, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yuan, Z.; Wu, H.; Liu, F.; Zhao, J. Molecular characterization of a manganese superoxide dismutase and copper/zinc superoxide dismutase from the mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2013, 34, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Umasuthan, N.; Bathige, S.D.N.K.; Thulasitha, W.S.; Qiang, W.; Lim, B.S.; Lee, J. Characterization of rock bream (Oplegnathus fasciatus) cytosolic Cu/Zn superoxide dismutase in terms of molecular structure, genomic arrangement, stress-induced mRNA expression and antioxidant function. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 176, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Sun, R.; Shi, W.; Yan, Y.; Li, H.; Guo, X.; Xu, H. Characterization of a mitochondrial manganese superoxide dismutase gene from Apis cerana cerana and its role in oxidative stress. J. Insect Physiol. 2014, 60, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Buswell, J.A. Mushroom nutriceuticals. World J. Microbiol. Biotechnol. 1996, 12, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.G. Indoor cultivation of paddy straw mushroom, Volvariella volvacea, in crates. Mycology 2000, 14, 174–176. [Google Scholar] [CrossRef]
- Zhao, F.; Lin, J.; You, L.; Guo, L. Exploration on industrialized cultivation pattern of Volvariella volvacea. Edible Fungi China 2010, 29, 26–27. (In Chinese) [Google Scholar]
- Chen, M.; Yu, Z.; Fan, P.; Ling, X.; Wang, N.; Tan, Q.; Lu, Z.; Pan, Y. Study on the Genetic Variation in tissue isolates of Volvariella volvacea. Acta Edulis Fungi 2000, 7, 11–14. (In Chinese) [Google Scholar]
- Liu, Z.; Zhang, K.; Lin, J.F.; Guo, L.Q. Breeding cold tolerance strain by chemical mutagenesis in Volvariella volvacea. Sci. Hortic. 2011, 130, 18–24. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z. A study on the superoxide dismutase of the fruitbody of straw mushroom, Volvariella volvacea. J. Fujian Agric. Coll. 1987, 16, 220–223. (In Chinese) [Google Scholar]
- Li, J.; Zhu, J.; Guo, S. The Superoxide Dismutase of the mycelia of straw mushroom, Volvariella volvacea. J. Fujian Agric. Coll. 1990, 19, 263–267. (In Chinese) [Google Scholar]
- Belinky, P.A.; Goldberg, D.; Krinfeld, B.; Burger, M.; Rothschild, N.; Cogan, U.; Dosoretz, C.G. Manganese-containing superoxide dismutase from the white-rot fungus Phanerochaete chrysosporium: Its function, expression and gene structure. Enzym. Microb. Technol. 2002, 31, 754–764. [Google Scholar] [CrossRef]
- Yin, C.; Zhao, W.; Zheng, L.; Chen, L.; Tan, Q.; Shang, X.; Ma, A. High-level expression of a manganese superoxide dismutase (PoMn-SOD) from Pleurotus ostreatus in Pichia pastoris. Appl. Biochem. Biotechnol. 2014, 174, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lamarre, C.; LeMay, J.D.; Deslauriers, N.; Bourbonnais, Y. Candida albicans expresses an unusual cytoplasmic manganese-containing superoxide dismutase (SOD3 gene product) upon the entry and during the stationary phase. J. Biol. Chem. 2001, 276, 43784–43791. [Google Scholar] [CrossRef] [PubMed]
- Fréalle, E.; Noël, C.; Nolard, N.; Symoens, F.; Felipe, M.S.; Dei-Cas, E.; Camus, D.; Viscogliosi, E.; Delhaes, L. Manganese superoxide dismutase based phylogeny of pathogenic fungi. Mol. Phylogenet. Evol. 2006, 41, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Lindström, B. Validity of Antonovsky’s sense of coherence scale: A systematic review. J. Epidemiol. Community Health 2005, 59, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Graveley, B.R. Alternative splicing: Increasing diversity in the proteomic world. Trends Genet. 2001, 17, 100–107. [Google Scholar] [CrossRef]
- Roberts, G.C.; Smith, C.W.J. Alternative splicing: Combinatorial output from the genome. Curr. Opin. Chem. Biol. 2002, 6, 375–383. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lai, Z.X. Superoxide dismutase multigene family in longan somatic embryos: A comparison of CuZn-SOD, Fe-SOD, and Mn-SOD gene structure, splicing, phylogeny, and expression. Mol. Breed. 2013, 32, 595–615. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, M.; Li, Y.; Tao, Y.; Jiang, Y.; Xie, B. Cloning and expression of 6-phosphogluconate dehydrogenase alternative splicing in Volvariella volvacea. Chin. J. Appl. Environ. Biol. 2014, 20, 584–589. (In Chinese) [Google Scholar]
- Tao, Y.; Zhang, L.; Guo, L.; Chen, R.; Lian, L.; Xie, B. Cloning and expression analysis of glucose-6-phosphate dehydrogenase gene and alternative splicing variant in Volvariella volvacea. Mycosystema 2015, 34, 724–733. (In Chinese) [Google Scholar]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular superoxide dismutase in biology and medicine. Free Radic. Biol. Med. 2003, 35, 236–256. [Google Scholar] [CrossRef]
- Lin, Y.C.; Vaseeharan, B.; Chen, J.C. Identification of the extracellular copper–zinc superoxide dismutase (ecCuZnSOD) gene of the mud crab Scylla serrata and its expression following β-glucan and peptidoglycan injections. Mol. Immunol. 2008, 45, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, E.D.; Edwards, J.A.; Youseff, B.H.; Rappleye, C.A. Definition of the extracellular proteome of pathogenic-phase Histoplasma capsulatum. J. Proteome Res. 2011, 10, 1929–1943. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Du, J.; Yao, W.; Xiu, Y.; Li, Y.; Gu, W.; Wang, W. An extracellular copper/zinc superoxide dismutase (ecCuZnSOD) from Chinese mitten crab, Eriocheir sinensis and its relationship with Spiroplasma eriocheiris. Aquaculture 2011, 320, 56–61. [Google Scholar] [CrossRef]
- Youseff, B.H.; Holbrook, E.D.; Smolnycki, K.A.; Rappleye, C.A. Extracellular superoxide dismutase protects Histoplasma yeast cells from host-derived oxidative stress. PLoS Pathog. 2012, 8, e1002713. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.; Wu, T.; Du, J.; Yao, W.; Li, W.; Ding, Z.; Ren, Q.; Gu, W.; Meng, Q.; Wang, W. Molecular characterization and expression analysis of extracellular copper/zinc superoxide dismutase (ecCuZnSOD) from oriental river prawn, Macrobrachium nipponense. Aquaculture 2013, 380, 23–28. [Google Scholar] [CrossRef]
- Bao, D.; Gong, M.; Zheng, H.; Chen, M.; Zhang, L.; Wang, H.; Jiang, J.; Wu, L.; Zhu, Y.; Zhu, G.; et al. Sequencing and comparative analysis of the straw mushroom (Volvariella volvacea) genome. PLoS ONE 2013, 8, e58294. [Google Scholar] [CrossRef] [PubMed]
- Takao, S.; Smith, E.H.; Wang, D.; Chan, C.K.; Bulkley, G.B.; Klein, A.S. Role of reactive oxygen metabolites in murine peritoneal macrophage phagocytosis and phagocytic killing. Am. J. Physiol. Cell Physiol. 1996, 40, C1278–C1284. [Google Scholar]
- Karlsson, M.; Stenlid, J.; Olson, Å. Identification of a superoxide dismutase gene from the conifer pathogen Heterobasidion annosum. Physiol. Mol. Plant Pathol. 2005, 66, 99–107. [Google Scholar] [CrossRef]
- Lambou, K.; Lamarre, C.; Beau, R.; Dufour, N.; Latge, J.P. Functional analysis of the superoxide dismutase family in Aspergillus fumigatus. Mol. Microbiol. 2010, 75, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Veluchamy, S.; Williams, B.; Kim, K.; Dickman, M.B. The CuZn superoxide dismutase from Sclerotinia sclerotiorum is involved with oxidative stress tolerance, virulence, and oxalate production. Physiol. Mol. Plant Pathol. 2012, 78, 14–23. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Oger, E.; Benabdellah, K.; Azcón-Aguilar, C.; Lanfranco, L.; Ferrol, N. Characterization of a CuZn superoxide dismutase gene in the arbuscular mycorrhizal fungus Glomus intraradices. Curr. Genet. 2010, 56, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Baek, Y.U.; Yim, H.S.; Kang, S.O. Protective roles of mitochondrial manganese containing superoxide dismutase against various stresses in Candida albicans. Yeast 2003, 20, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Yan, Y.Z.; Shen, R.D. Meiosis and behavior of nuclei during basidiospore formation in Volvariella volvacea. Acta Microbiol. Sin. 1991, 10, 72–78. (In Chinese) [Google Scholar]
- Sun, X.; Feng, A.; Chen, M.; Pan, Y.J. Cloning and sequence analysis of cold induced genes in Chinese straw mushroom, Volvariella volvacea. Mycosystema 2005, 25, 88–93. (In Chinese) [Google Scholar]
- Wang, S.; Zhang, H.; Cheng, Q.; He, Q.; He, H. Effect of chilling stress on the expression of antioxidative enzymes in Volvariella volvacea mycelia. Chin. J. Trop. Crop. 2009, 30, 593–597. (In Chinese) [Google Scholar]
- Wu, D.F.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar] [PubMed]
- Li, R.; Zhu, H.; Ruan, J.; Qian, W.; Fang, X.; Shi, Z.; Li, Y.; Li, S.; Shan, G.; Kristiansen, K.; et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010, 20, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.; Yan, J.; Xie, B.; Li, Y.; Chen, B.; Liu, S.; Li, D.; Yang, Z.; Zeng, X.; Deng, Y.; et al. Genes encoding FAD-binding proteins in Volvariella volvacea exhibit differential expression in homokaryons and heterokaryons. Microbiol. Res. 2013, 168, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, L.; Zhao, J.; Xie, B. Sequence characterization and differential expression of a glutathione S-transferase gene vv-gto1 from Volvariella volvacea. Acta Microbiol. Sin. 2014, 54, 1171–1177. (In Chinese) [Google Scholar]
- Zhang, Z.; Lin, H.; Ma, B. ZOOM Lite: Next-generation sequencing data mapping and visualization software. Nucleic Acids Res. 2010, 38, W743–W748. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, K.B.; Nicholas, H.B., Jr.; Deerfield, D.W., II. GeneDoc: Analysis and visualization of genetic variation. Embnet. News 1997, 4, 1–4. [Google Scholar]
- Claros, M.G.; Vincens, P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 1996, 241, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ge, W.; Buswell, J.A. Molecular cloning of a new laccase from the edible straw mushroom Volvariella volvacea: Possible involvement in fruit body development. FEMS Microbiol. Lett. 2004, 230, 171–176. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, B.; Yang, Z.; Chen, Z.; Chen, B.; Deng, Y.; Jiang, Y.; van Peer, A.F. Identification and expression analysis of a new glycoside hydrolase family 55 exo-β-1,3-glucanase-encoding gene in Volvariella volvacea suggests a role in fruiting body development. Gene 2013, 527, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.-J.; Zhang, L.; Wang, R.-Q.; Xie, B.; Li, X.; Chen, R.-L.; Guo, L.-X.; Xie, B.-G. The Sequence Characteristics and Expression Models Reveal Superoxide Dismutase Involved in Cold Response and Fruiting Body Development in Volvariella volvacea. Int. J. Mol. Sci. 2016, 17, 34. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010034
Yan J-J, Zhang L, Wang R-Q, Xie B, Li X, Chen R-L, Guo L-X, Xie B-G. The Sequence Characteristics and Expression Models Reveal Superoxide Dismutase Involved in Cold Response and Fruiting Body Development in Volvariella volvacea. International Journal of Molecular Sciences. 2016; 17(1):34. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010034
Chicago/Turabian StyleYan, Jun-Jie, Lei Zhang, Rui-Qing Wang, Bin Xie, Xiao Li, Ren-Liang Chen, Li-Xian Guo, and Bao-Gui Xie. 2016. "The Sequence Characteristics and Expression Models Reveal Superoxide Dismutase Involved in Cold Response and Fruiting Body Development in Volvariella volvacea" International Journal of Molecular Sciences 17, no. 1: 34. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010034