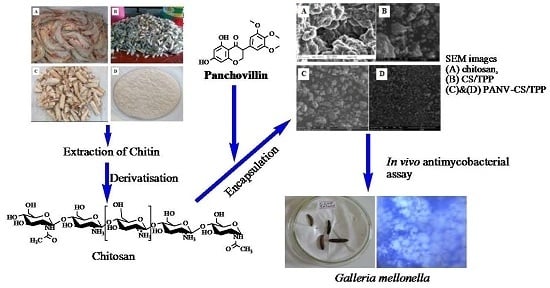
Preparation, Characterization and in Vivo Antimycobacterial Studies of Panchovillin-Chitosan Nanocomposites
Abstract
:1. Introduction
2. Results and Discussion
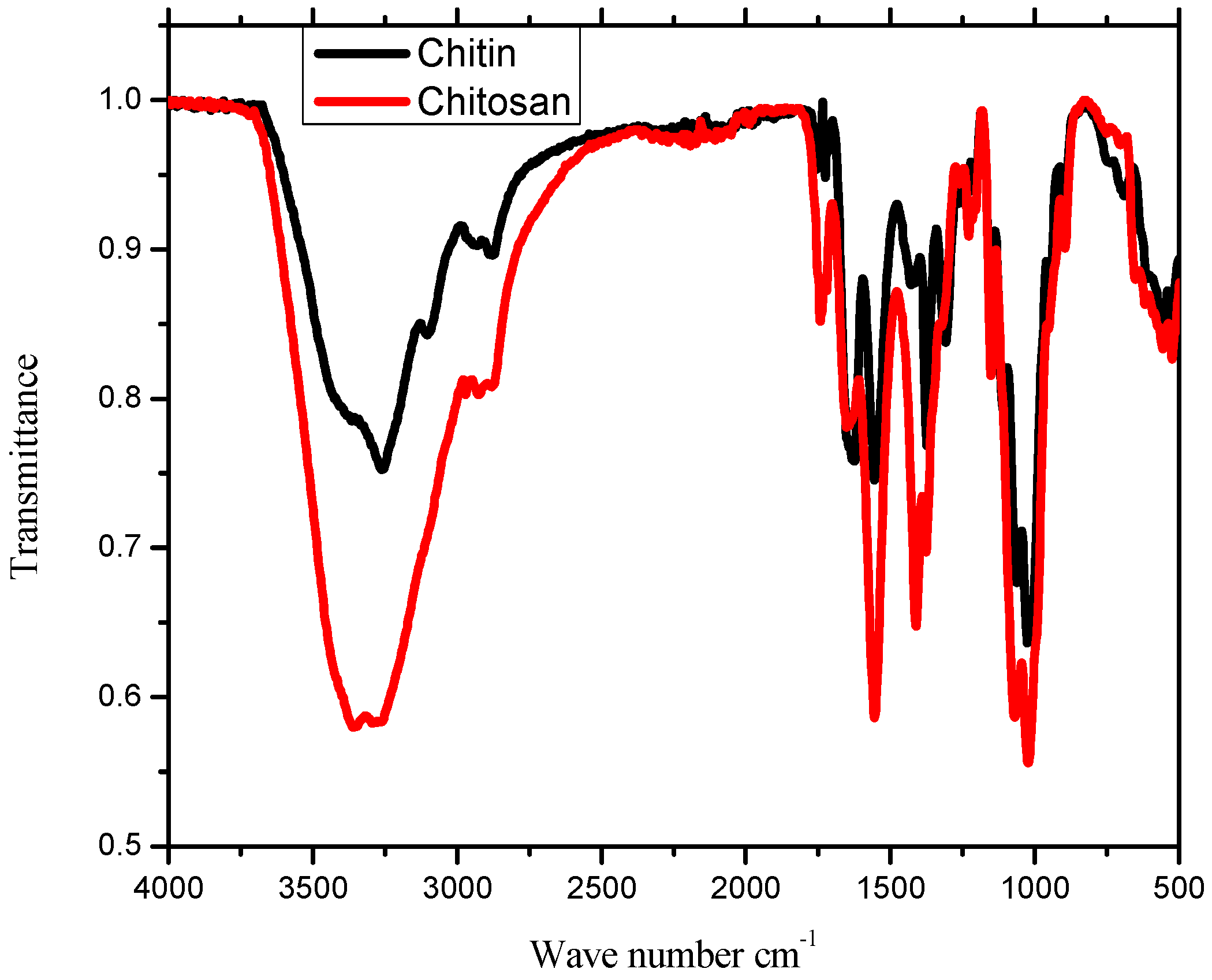
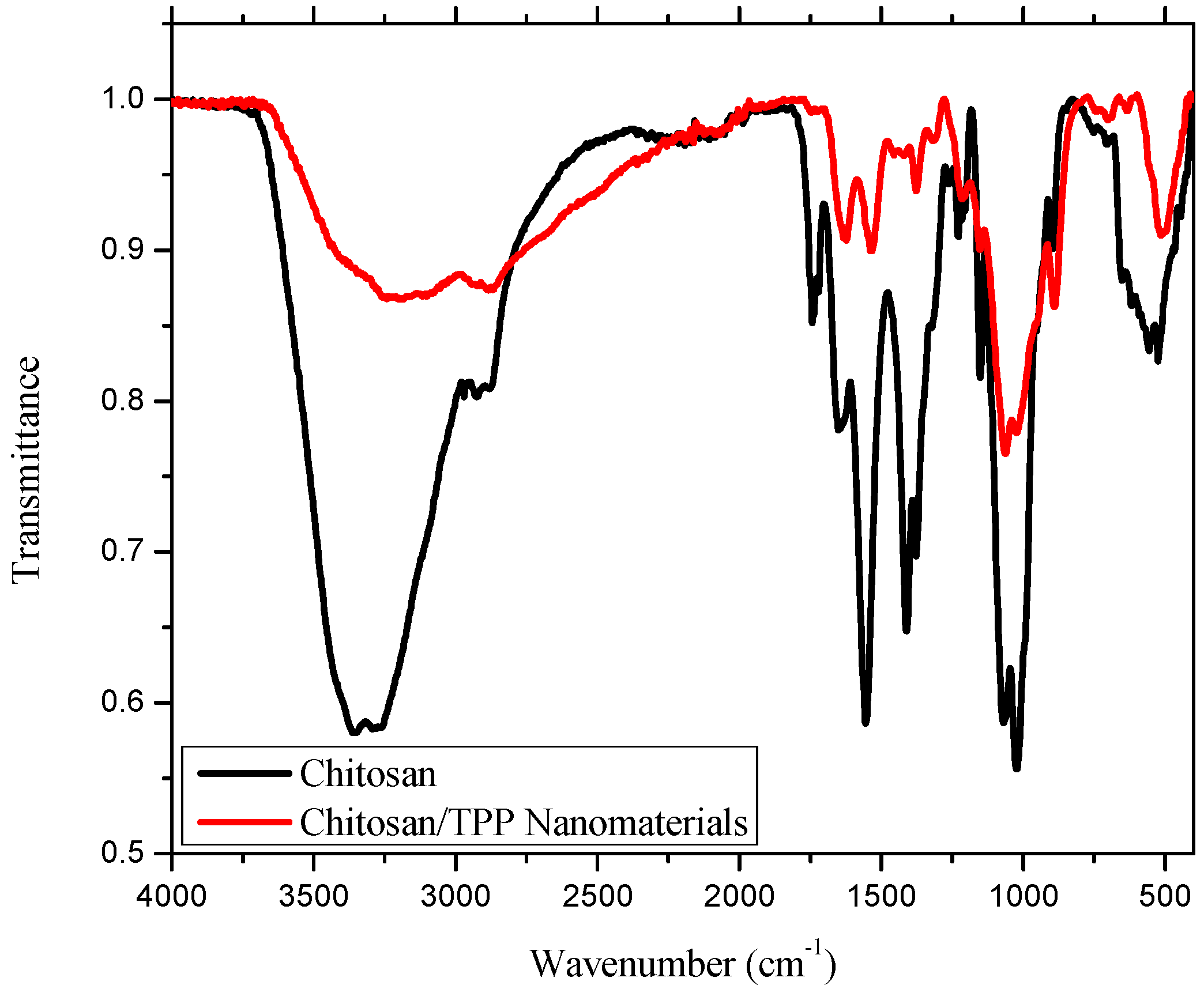
2.1. IR Analysis
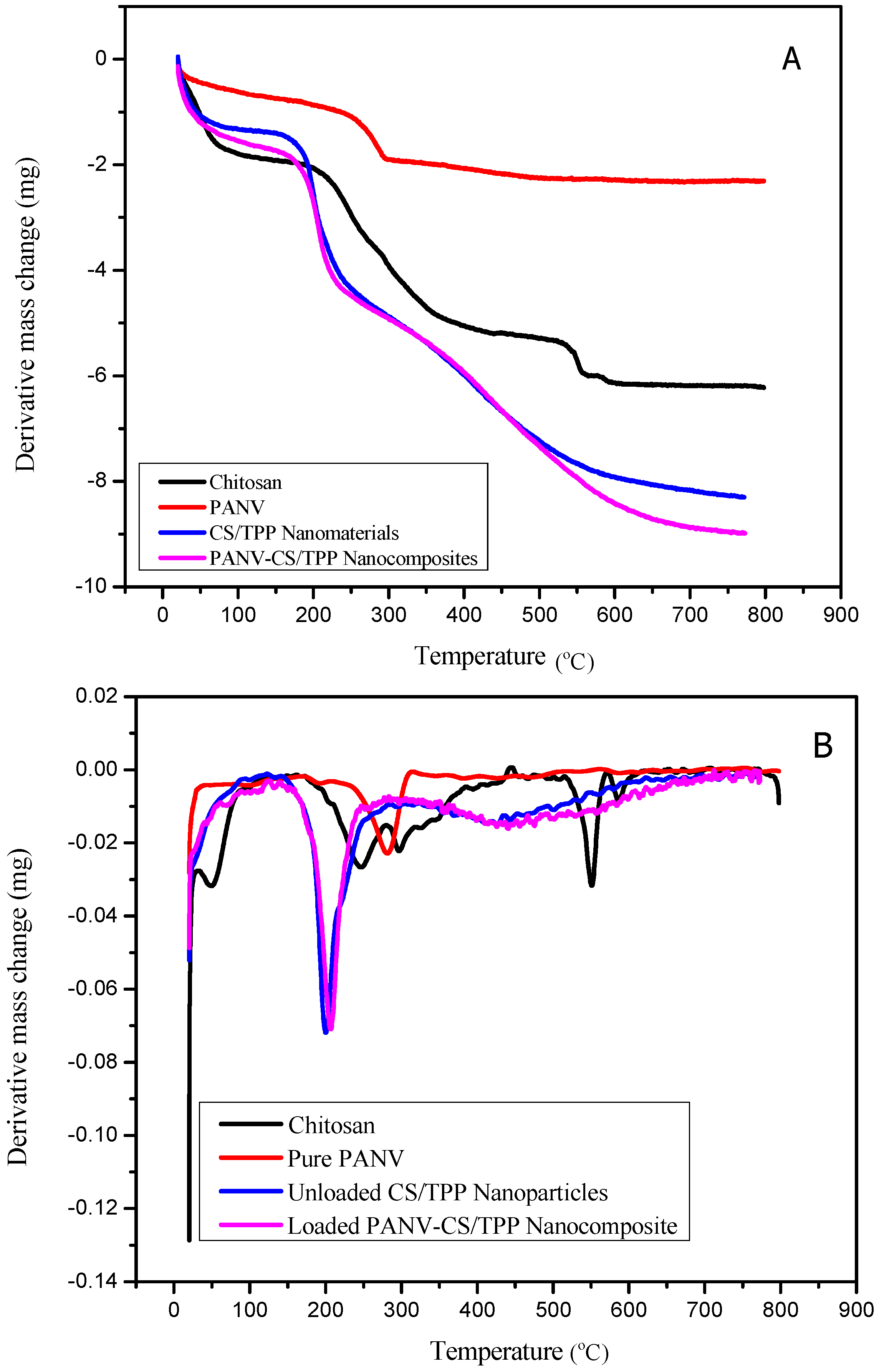
2.2. Thermal Analysis
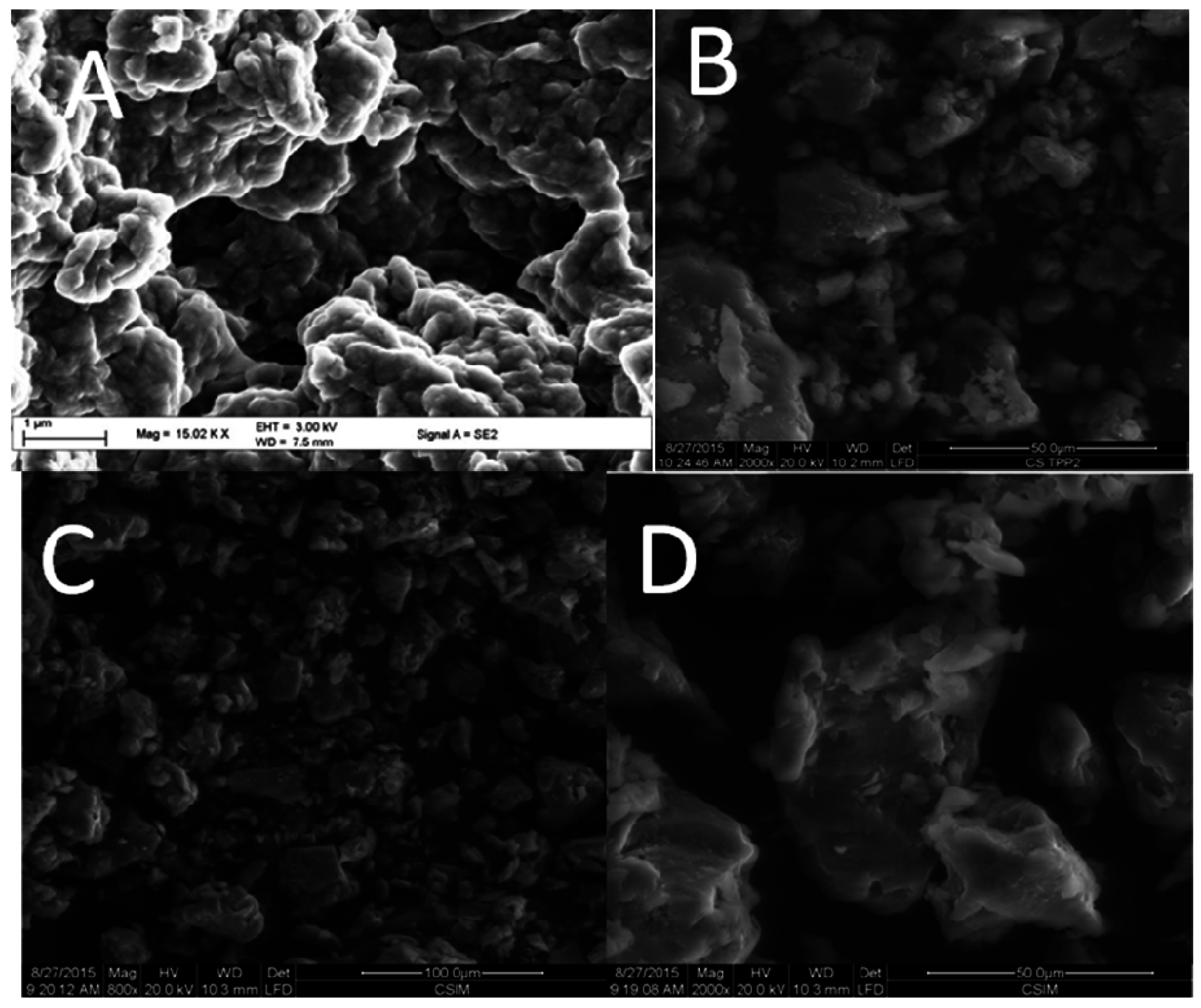
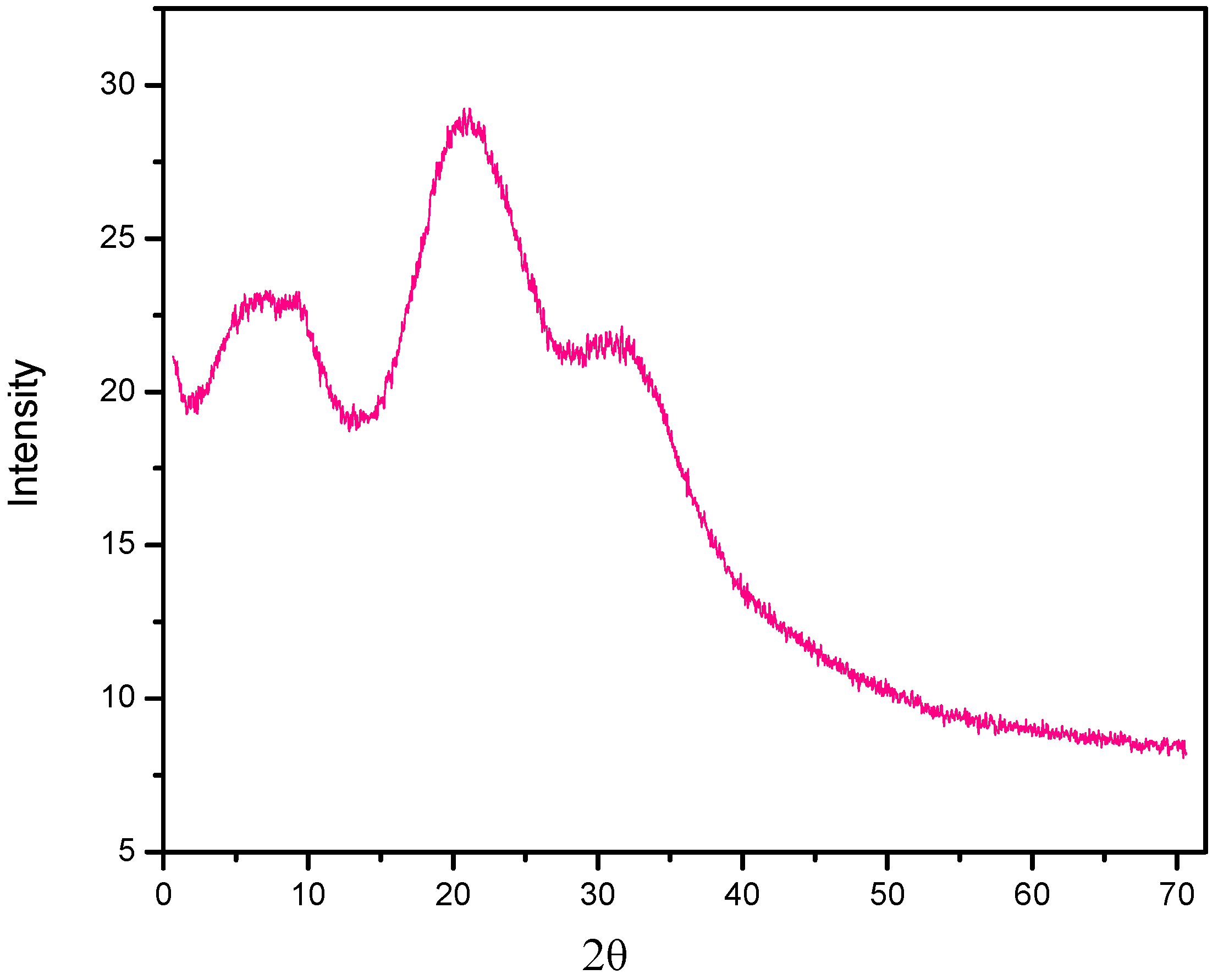
2.3. SEM and X-ray Diffraction
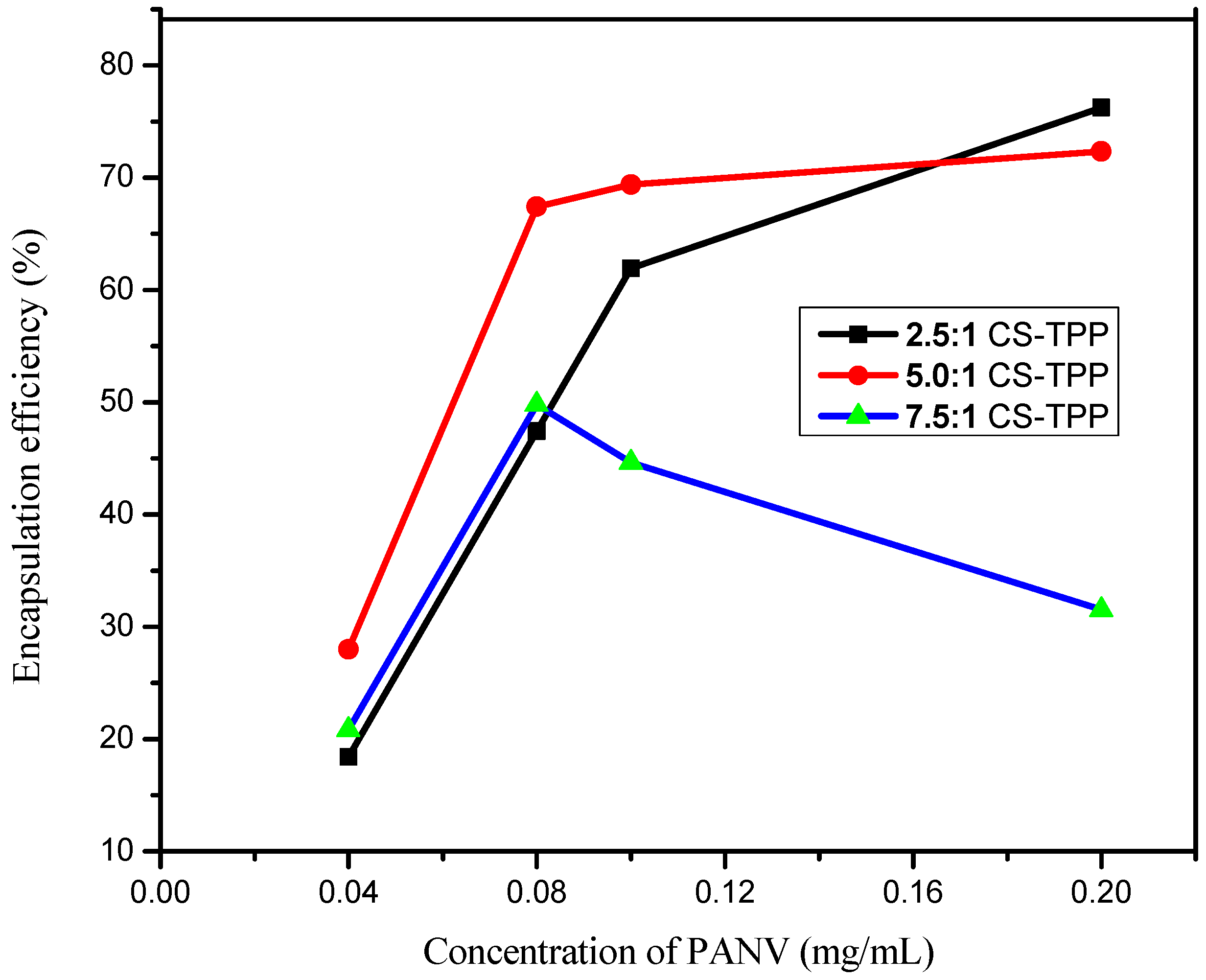
2.4. Effect of PANV and Chitosan Concentrations on Encapsulation Efficiency
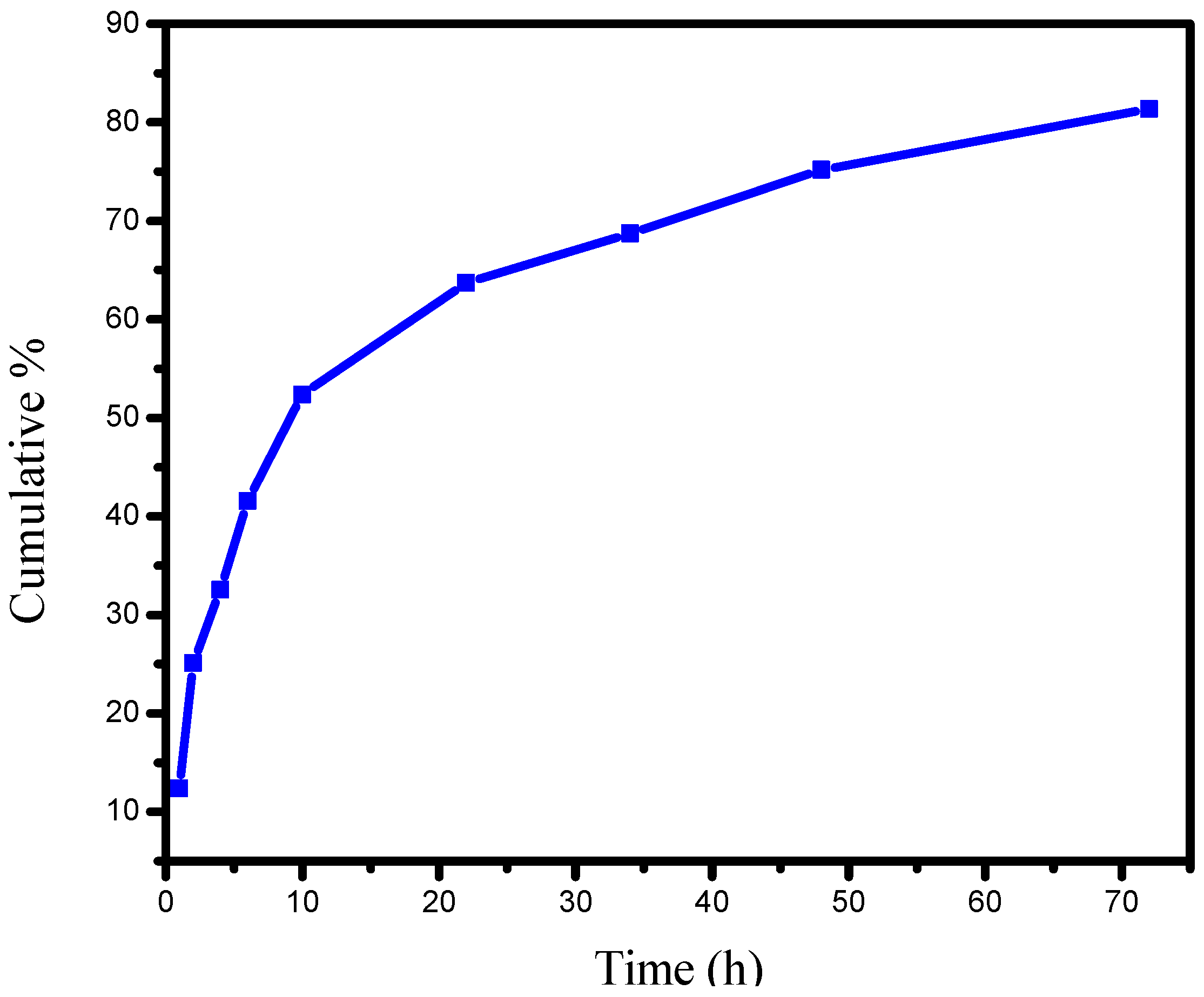
2.5. In Vitro Release Studies of PANV-CS/TPP
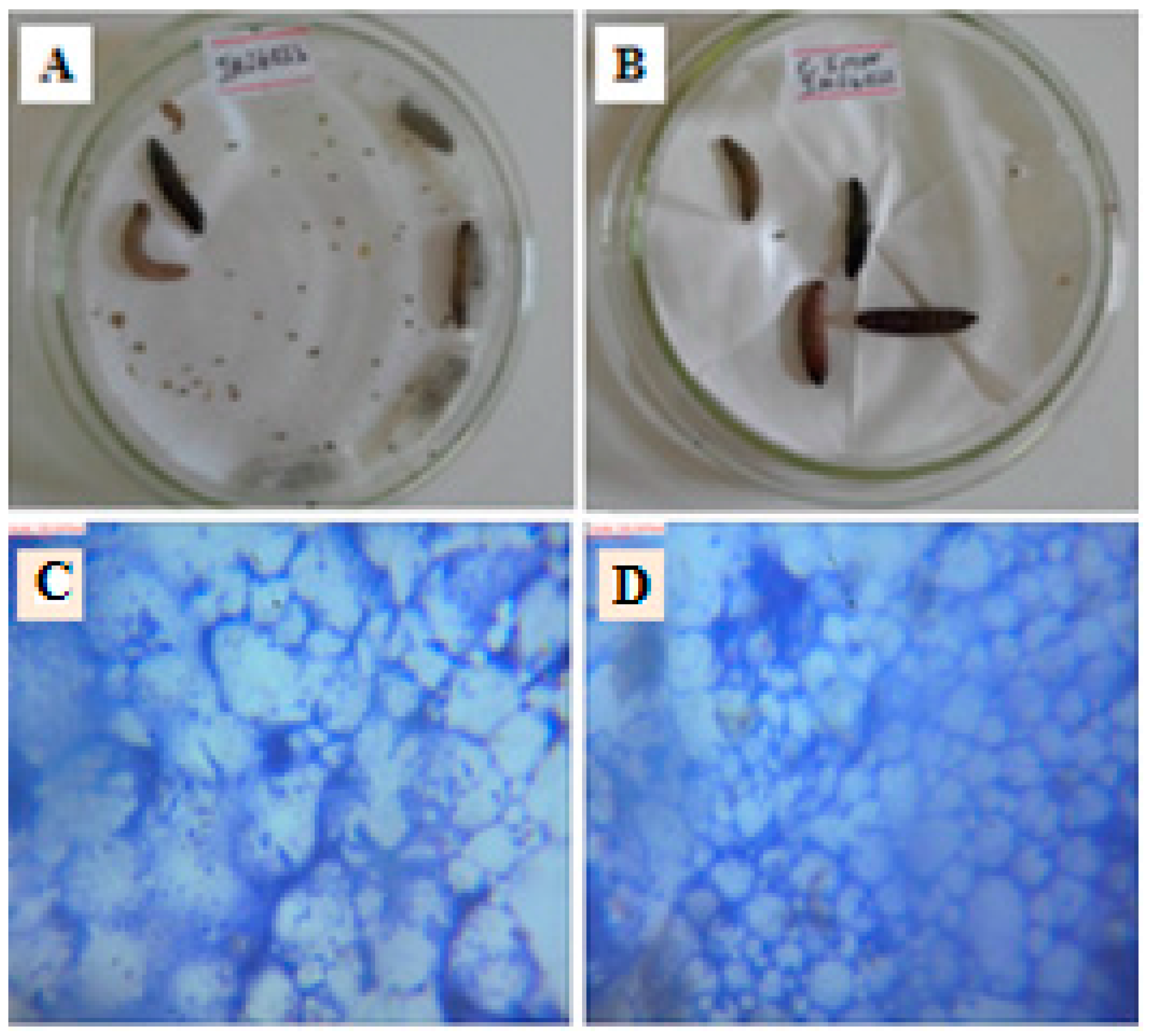
2.6. In Vivo Antimycobacterial Assay
3. Materials and Methods
3.1. Test Organisms
3.2. Reagents and Other Materials
3.3. Sample Collection
3.4. Purification of Chitin
3.5. Preparation of Chitosan by N-Deacetylation of Chitin
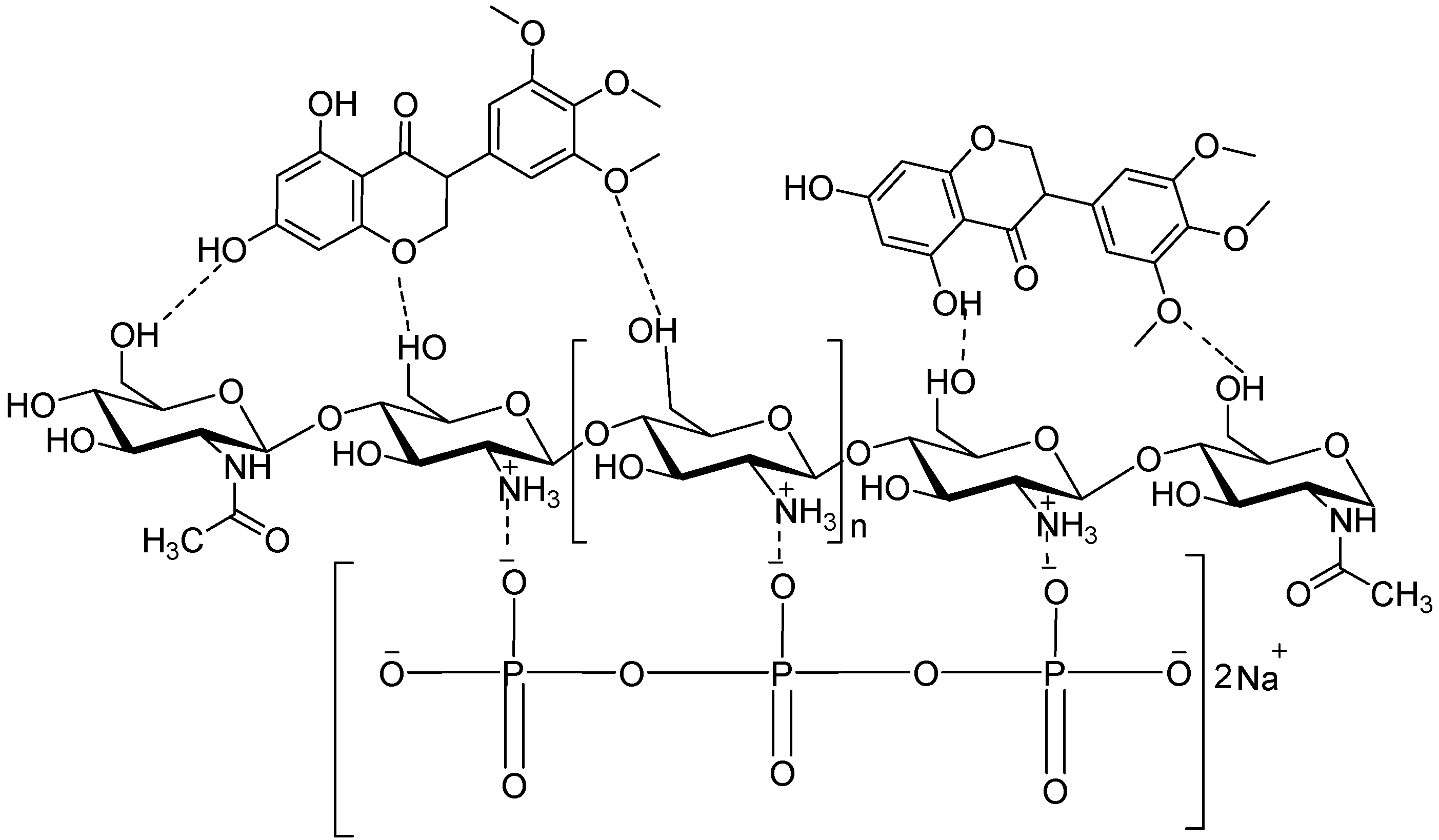
3.6. Preparation of CS/TPP and PANV-CS/TPP Nanocomposites
3.7. Effect of PANV and Chitosan Concentrations on Encapsulation Efficiency
3.8. Encapsulation of PANV for Bioassay Using Chitosan Solution
3.9. Encapsulation Efficiency and Loading Capacity
3.10. In Vitro Release Studies
3.11. In Vivo Antimycobacterial Bioassay Using Galleria Mellonella
3.12. Characterization of Materials
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bharali, D.J.; Siddiqui, I.A.; Adhami, V.M.; Chamcheu, J.C.; Aldahmash, A.M.; Mukhtar, H.; Mousa, S.A. Nanoparticle delivery of natural products in the prevention and treatment of cancers: Current status and future prospects. Cancers 2011, 3, 4024–4045. [Google Scholar] [CrossRef] [PubMed]
- Ranjita, S.; Loaye, A.; Khalil, M. Present status of nanoparticle research for treatment of tuberculosis. J. Pharm. Sci. 2011, 14, 100–116. [Google Scholar]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.A. Novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Ochekpe, N.A.; Olorunfemi, P.O.; Ngwuluka, N.C. Nanotechnology and drug delivery part 2: Nanostructures for drug delivery. Trop. J. Pharm. Res. 2009, 8, 275–287. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Sarma, S.J.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Recent development in applications of important biopolymer chitosan in biomedicine, pharmaceuticals and personal care products. Curr. Tissue Eng. 2013, 2, 20–40. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S.; Tao, Y.; Zang, L.; Su, Z. Preparation and characterization of water soluble chitosan nanoparticles as protein delivery system. J. Nanomater. 2009, 2010, 1–6. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report. 2013. Available online: http://www.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf (accessed on 1 April 2015).
- Thangapazham, R.L.; Puri, A.; Tele, S.; Blumenthal, R.; Maheshwari, R.K. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008, 32, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.; Coy, E.D.; Stashenko, E. Antimycobacterial natural products—An opportunity for the colombian biodiversity. Rev. Esp. Quimioter. 2011, 24, 175–183. [Google Scholar] [PubMed]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef]
- Clarke, G.P.; Burgess, N.D.; Mbago, F.M.; Mligo, C.; Mackinder, B.; Gereau, R.E. Two “extinct” trees rediscovered near Kilwa, Tanzania. J. East Afr. Nat. Hist. 2011, 100, 133–140. [Google Scholar] [CrossRef]
- Nyandoro, S.S.; Munissi, J.J.E.; Kombo, M.; Mgina, C.A.; Fangfang, P.; Gruhonjic, A.; Fitzpatrick, P.; Landberg, G.; Lu, Y.; Wang, B.; et al. Flavonoids from Erythrina schliebenii. J. Nat. Prod. 2016. under preparation. [Google Scholar]
- Adochitei, A.; Drochioiu, G. Rapid Characterization of peptide secondary structure by FT-IR spectroscopy. Rev. Roum. Chim. 2010, 56, 783–791. [Google Scholar]
- Kamala, K.; Sivaperumal, P.; Rajaram, R. Extraction and characterization of water soluble chitosan from parapeneopsis stylifera shrimp shell waste and its antibacterial activity. Int. J. Sci. Res. Pub. 2013, 3, 1–8. [Google Scholar]
- Ujang, Z.; Diah, M.; Rashid, A.H.A.; Halim, A.S. The development, characterization and application of water soluble chitosan. Biotechnol. Biopolym. 2011, 2011, 1–23. [Google Scholar]
- Zhang, A.; Qin, Q.; Zhang, H.; Wang, H.; Li, X.; Miao, L.; Wu, Y. Preparation and characterisation of food-grade chitosan from housefly larvae. Czech. J. Food. Sci. 2011, 29, 616–623. [Google Scholar]
- Wanule, D.; Balkhande, J.V.; Ratnakar, P.U.; Kulkarni, A.N.; Bhowate, C.S. Extraction and ftir analysis of chitosan from american cockroach, periplaneta americana. Int. J. Eng. Sci. Innov. Technol. 2014, 3, 299–304. [Google Scholar]
- Mohanasrinivasan, V.; Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Suganthi, V.; Devi, C.S. Studies on heavy metal removal efficiency and antibacterial activity of chitosan prepared from shrimp shell waste. J. Biotechnol. 2014, 4, 167–175. [Google Scholar] [CrossRef]
- Mohammadpour, D.N.; Eskandari, R.; Avadi, M.R.; Zolfagharian, H.; Mir Mohammad, S.A.; Rezayat, M. Preparation and in vitro characterization of chitosan nanoparticles containing Mesobuthus eupeus scorpion venom as an antigen delivery system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 44–52. [Google Scholar]
- Vimal, S.; Taju, G.; Nambi, K.S.N.; Majeed, S.A.; Babu, V.S.; Ravi, M.; Hameed, A.S.S. Synthesis and characterization of CS/TPP nanoparticles for oral delivery of gene in fish. Aquacult 2012, 2012, 14–22. [Google Scholar] [CrossRef]
- Peniche-Covas, C.; Argiielles-Monai, W.; Roman, S.J. A kinetic study of the thermal degradation of chitosan and a mercaptan derivative of chitosan. Polym. Degrad. Stab. 1993, 39, 21–28. [Google Scholar] [CrossRef]
- Neto, C.G.T.; Giacometti, J.A.; Job, A.E.; Ferreira, F.C.; Fonseca, J.L.C.; Pereira, M.R. Thermal analysis of chitosan based networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Elhefian, E.A.; Nasef, M.M.; Yahaya, A.H. Preparation and characterization of chitosan/agar blended films: Part 2. Thermal, mechanical, and surface properties. J. Chem. 2012, 9, 510–516. [Google Scholar] [CrossRef]
- Puchalska, A.; Mucha, M. Thermogravimetry of chitosan with nanofillers. Prog. Chem. Appl. Chitin 2011, 16, 31–42. [Google Scholar]
- Pieróg, M.; Czubenko, J.O.; Drużyńska, M.G. Thermal degradation of double crosslinked hydrogel chitosan membranes. Prog. Chem. Appl. Chitin 2012, 17, 67–74. [Google Scholar]
- Gallego, R.; Arteaga, J.F.; Valencia, C.; Franco, J.M. Isocyanate-functionalized chitin and chitosan as gelling agents of castor oil. Molecules 2013, 18, 6532–6549. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Muthunarayanan, M.; Muthuchelian, K.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Saponin-loaded chitosan nanoparticles and their cytotoxicity to cancer cell lines in vitro. Carbohydr. Polym. 2010, 84, 407–416. [Google Scholar] [CrossRef]
- Parida, U.K.; Rout, N.; Bindhani, B.K. In vitro properties of chitosan nanoparticles induce apoptosis in human lymphoma SUDHL-4 cell line. Adv. Biosci. Biotechnol. 2013, 4, 1118–1127. [Google Scholar] [CrossRef]
- Pandey, R.; Khuller, G.K. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis 2005, 85, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Stoica, R.; Şomoghi, R.; Ion, R.M. Preparation of chitosan-tripolyphosphate nanoparticles the encapsulation of polyphenols extracted from rose hips. Dig. J. Nanomater. Biostruct. 2013, 8, 955–963. [Google Scholar]
- Cook, S.M.; McArthur, J.D. Developing Galleria mellonella as a model host for human pathogens. Med. Health Sci. 2013, 4, 350–353. [Google Scholar]
- Abourashed, E.A. Bioavailability of plant-derived antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Masum, S.M.; Rahman, M.M.; Molla, M.A.I.; Shaikh, A.A.; Roy, S.K. Preparation of chitosan from shrimp shell and investigation of its properties. Int. J. Basic Appl. Sci. 2011, 11, 116–130. [Google Scholar]
- Ploydee, E.; Chaiyanan, S. Production of high viscosity chitosan from biologically purified chitin isolated by microbial fermentation and deproteinization. Int. J. Polym. Sci. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Akhtar, N.; Tahir, H.; Sultan, M.; Yasmeen, G.; Hameed, U. Application of chitosan padded rice and wheat husk for the removal of reactive dye from aqueous solution. Afr. J. Biotechnol. 2012, 11, 2756–12765. [Google Scholar]
- Puvvada, Y.S.; Vankayalapat, S.; Sukhavasi, S. Extraction of chitin and chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. Int. Curr. Pharm. J. 2012, 1, 258–263. [Google Scholar] [CrossRef]
- Mirzadehl, H.; Yaghobi, N.; Amanpour, S.; Ahmadi, H.; Mohagheghi, M.A.; Hormozi, F. Preparation of chitosan derived from shrimp’s shell of persian gulf as a blood hemostasis agent. Iran Polym. J. 2002, 11, 63–68. [Google Scholar]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan–polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Vimal, S.; Majeed, S.A.; Taju, G.; Nambi, K.S.N.; Raj, N.S.; Madan, N.; Farook, M.A.; Rajkumar, T.; Gopinath, D.; Hameed, A.S.S. Chitosan tripolyphosphate (CS/TPP) nanoparticles: Preparation, characterization and application for gene delivery in shrimp. Acta Trop. 2013, 128, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shi, L.; Zhang, Z.; Chen, J.; Shi, D.; Yang, J.; Tang, Z. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng. 2011, 28, 353–362. [Google Scholar] [CrossRef]
- Ponnuraj, R.; Janakiraman, K.; Gopalakrishnan, S.; Senthilnathan, K.; Meganathan, V.; Saravanan, P. Formulation and characterization of epigallocatechin gallate nanoparticles. Am. J. Pharm. Res. 2015, 5, 387–399. [Google Scholar]
- Cover, N.F.; Yuen, S.L.; Parsons, A.K.; Kumar, A. Synergetic Effects of doxycycline-loaded chitosan nanoparticles for improving drug delivery and efficacy. Int. J. Nanomed. 2012, 7, 2411–2419. [Google Scholar]
- Nathiya, S.; Durga, M.; Devasena, T. Preparation, physico-chemical characterization and biocompatibility evaluation of quercetin loaded chitosan nanoparticles and its novel potential to ameliorate monocrotophos induced toxicity. Dig. J. Nanomater. Biostruct. 2014, 9, 1603–1614. [Google Scholar]
- Anbarasan, B.; Menon, V.V.; Niranjana, V.A.; Ramaprabhu, S. Optimization of the formulation and in-vitro evaluation of chloroquine loaded chitosan nanoparticles using ionic gelation method. J. Chem. Pharm. Sci. 2013, 6, 106–112. [Google Scholar]
- Manikkam, R.; Pitchai, D. Catechin loaded chitosan nanoparticles as a novel drug delivery system for cancer–synthesis and in vitro and in vivo characterization. World J. Pharm. Pharm. Sci. 2013, 3, 1553–1577. [Google Scholar]
- Kasoju, N.; Bora, U. Fabrication and characterization of curcumin-releasing silk fibroin scaffold. J. Biomed. Mater. Res. B 2012, 100B, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Erasto, P. Antimycobacterial sterols from aromatic stem sap of Commiphora eminii. Engl. J. Adv. Sci. Res. 2012, 3, 27–31. [Google Scholar]
- Khan, T.A. Reporting degree of deacetylation values of chitosan: The influence of analytical methods. J. Pharm. Sci. 2002, 5, 205–212. [Google Scholar]
- Shadrack, D.M.; Mubofu, E.B.; Nyandoro, S.S. Synthesis of polyamidoamine dendrimer for encapsulating tetramethylscutellarein for potential bioactivity enhancement. Int. J. Mol. Sci. 2015, 16, 26363–26377. [Google Scholar] [CrossRef] [PubMed]



| Compound Dose | 24 h | 48 h | ||
|---|---|---|---|---|
| Dead (%) | Alive (%) | Dead (%) | Alive (%) | |
| PANV (80 nM/mg bw *) | 14 | 86 | 29 | 71 |
| PANV-CS/TPP (0.289 nM/mg bw *) | 20 | 80 | 60 | 40 |
| CS/TPP | 0 | 100 | 0 | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rwegasila, E.; Mubofu, E.B.; Nyandoro, S.S.; Erasto, P.; Munissi, J.J.E. Preparation, Characterization and in Vivo Antimycobacterial Studies of Panchovillin-Chitosan Nanocomposites. Int. J. Mol. Sci. 2016, 17, 1559. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101559
Rwegasila E, Mubofu EB, Nyandoro SS, Erasto P, Munissi JJE. Preparation, Characterization and in Vivo Antimycobacterial Studies of Panchovillin-Chitosan Nanocomposites. International Journal of Molecular Sciences. 2016; 17(10):1559. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101559
Chicago/Turabian StyleRwegasila, Edward, Egid B. Mubofu, Stephen S. Nyandoro, Paul Erasto, and Joan J. E. Munissi. 2016. "Preparation, Characterization and in Vivo Antimycobacterial Studies of Panchovillin-Chitosan Nanocomposites" International Journal of Molecular Sciences 17, no. 10: 1559. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101559