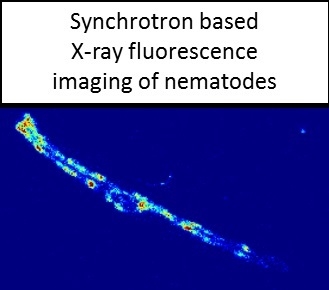
Deletion of Phytochelatin Synthase Modulates the Metal Accumulation Pattern of Cadmium Exposed C. elegans
Abstract
:1. Introduction
2. Results and Discussion
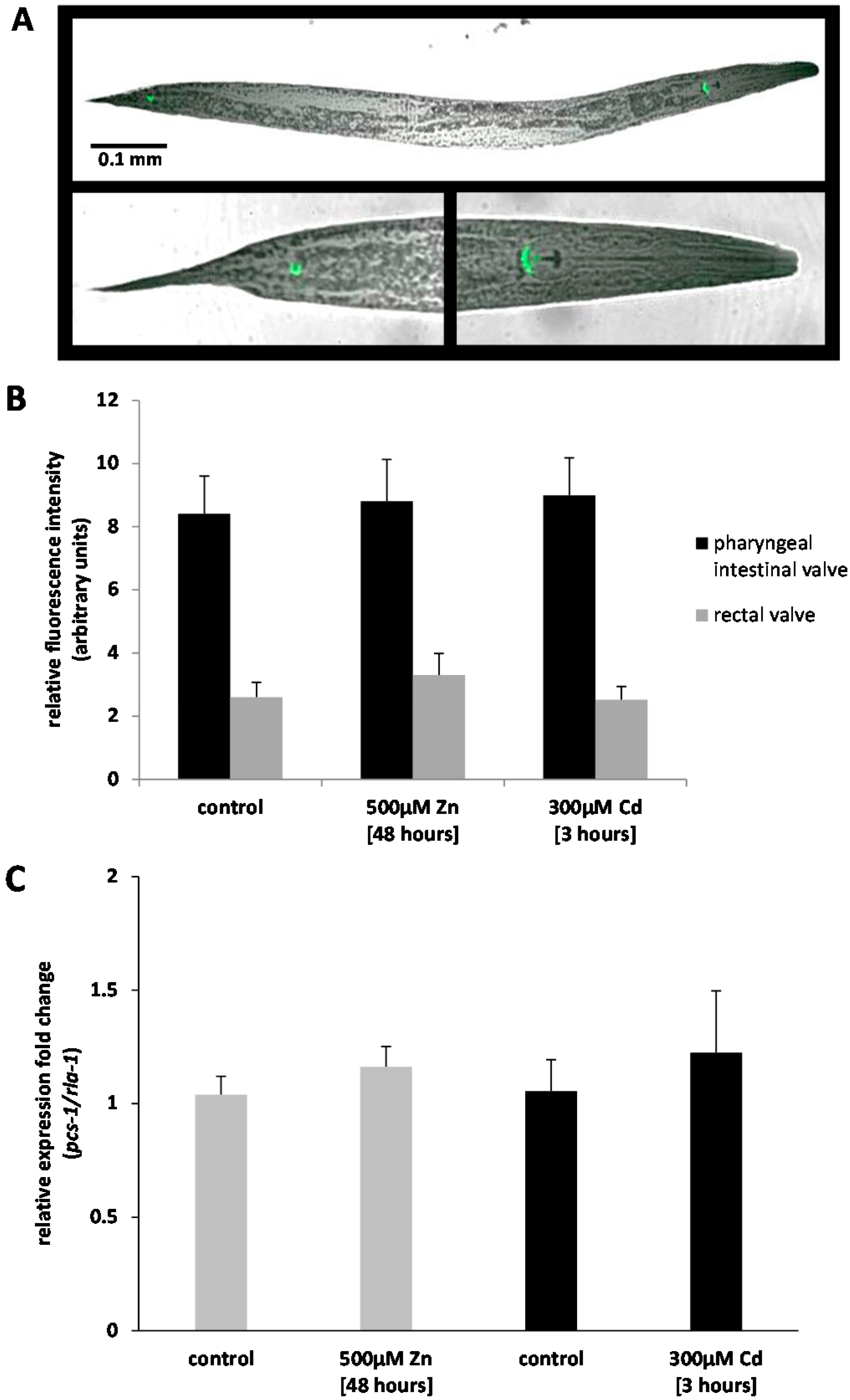
2.1. Analysis of Ppcs-1::GFP Expression by Fluorescence Microscopy
2.2. Analysis of Pcs-1 Expression by Quantitative PCR
2.3. Analysis of Metal Accumulation by X-ray Fluorescence Imaging
3. Materials and Methods
3.1. Maintenance and Strains
3.2. Metal Exposures
3.3. Confocal Fluorescence Microscopy
3.4. Quantitative PCR (qPCR)
3.5. X-ray Fluorescence Imaging (XFI)
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science 1985, 230, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Loffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef] [PubMed]
- Rea, P.A.; Vatamaniuk, O.K.; Rigden, D.J. Weeds, worms, and more. Papain’s long-lost cousin, phytochelatin synthase. Plant Physiol. 2004, 136, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Schroeder, J.I.; Degenkolb, T. Caenorhabditis elegans expresses a functional phytochelatin synthase. Eur. J. Biochem. 2001, 268, 3640–3643. [Google Scholar] [CrossRef] [PubMed]
- Vatamaniuk, O.K.; Bucher, E.A.; Ward, J.T.; Rea, P.A. A new pathway for heavy metal detoxification in animals. Phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 2001, 276, 20817–20820. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.L.; Bundy, J.G.; Want, E.J.; Kille, P.; Sturzenbaum, S.R. The metabolomic responses of Caenorhabditis elegans to cadmium are largely independent of metallothionein status, but dominated by changes in cystathionine and phytochelatins. J. Proteom. Res. 2009, 8, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.G.; Kille, P. Metabolites and metals in Metazoa—What role do phytochelatins play in animals? Metallomics 2014, 9, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Howden, R.; Andersen, C.R.; Goldsbrough, P.B.; Cobbett, C.S. A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol. 1995, 107, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. The metals in the biological periodic system of the elements: Concepts and conjectures. Int. J. Mol. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, M.M.; Moniuszko-Jakoniuk, J. Interactions between cadmium and zinc in the organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef]
- Cui, Y.; McBride, S.; Boyd, W.; Alper, S.; Freedman, J. Toxicogenomic analysis of Caenorhabditis elegans reveals novel genes and pathways involved in the resistance to cadmium toxicity. Genome Biol. 2007, 8, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Hope, I.A.; Stevens, J.; Garner, A.; Hayes, J.; Cheo, D.L.; Brasch, M.A.; Vidal, M. Feasibility of genome-scale construction of promoterreporter gene fusions for expression in Caenorhabditis elegans using a multisite gateway recombination system. Genome Res. 2004, 14, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.S.; Benci, J.L.; Selote, D.S.; Sharma, A.K.; Chen, A.G.; Dang, H.; Fares, H.; Vatamaniuk, O.K. Detoxification of multiple heavy metals by a half-molecule ABC transporter, HMT-1, and coelomocytes of Caenorhabditis elegans. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Vatamaniuk, O.K.; Mari, S.; Lu, Y.P.; Rea, P.A. AtPCS1, a phytochelatin synthase from Arabidopsis: Isolation and in vitro reconstitution. Proc. Natl. Acad. Sci. USA 1999, 96, 7110–7115. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Kim, E.J.; Neumann, D.; Schroeder, J.I. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999, 18, 3325–3333. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.B.; Smith, A.P.; Howden, R.; Dietrich, W.M.; Bugg, S.; O'Connell, M.J.; Goldsbrough, P.B.; Cobbett, C.S. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 1999, 11, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA 1987, 84, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Polak, N.; Read, D.S.; Jurkschat, K.; Matzke, M.; Kelly, F.J.; Spurgeon, D.J.; Sturzenbaum, S.R. Metalloproteins and phytochelatin synthase may confer protection against zinc oxide nanoparticle induced toxicity in Caenorhabditis elegans. Comp. Biochem. Physiol. C 2014, 160, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Swain, S.C.; Keusekotten, K.; Baumeister, R.; Stürzenbaum, S.R. C. elegans metallothioneins: New insights into the phenotypic effects of cadmium toxicosis. J. Mol. Biol. 2004, 341, 951–959. [Google Scholar] [CrossRef] [PubMed]

| Phylum Nematoda (Nematodes) | |||
| Class | Order | Species | Accession number |
| Chromadorea | Rhabditida | Caenorhabditis elegans | AF299333.1 |
| Chromadorea | Rhabditida | Pristionchus pacificus | ABKE02006821 |
| Chromadorea | Spirurida | Loa loa | XM_003138991.1 |
| Chromadorea | Ascaridida | Ascaris suum | AEUI02000059.1 |
| Secernentea | Rhabditida | Caenorhabditis briggsae | FR847113.2 |
| Secernentea | Rhabditida | Caenorhabditis remanei | DS268408.1 |
| Secernentea | Rhabditida | Caenorhabditis brenneri | XM_003117257.1 |
| Secernentea | Rhabditida | Haemonchus contortus | HF966434.1 |
| Secernentea | Strongylida | Ancylostoma ceylanicum | KE125293.1 |
| Secernentea | Strongylida | Necator americanus | XM_013449818.1 |
| Secernentea | Strongylida | Ancylostoma duodenale | KN734493.1 |
| Secernentea | Strongylida | Oesophagostomum dentatum | KN567239.1 |
| Secernentea | Strongylida | Ancylostoma ceylanicum | KC914882 |
| Secernentea | Spirurida | Brugia malayi | XM_001902065.1 |
| Secernentea | Ascaridida | Toxocara canis | JPKZ01001678.1 |
| Phylum Annelida (Segmented Worms) | |||
| Class | Order | Species | Accession number |
| Clitellata | Haplotaxida | Lumbricus rubellus | KC981075.1 / KC981074.1 |
| Clitellata | Haplotaxida | Eisenia fetida | EF433776.1 |
| Clitellata | Haplotaxida | Eisenia andrei | KP770990.1 |
| Clitellata | Rhynchobdellida | Helobdella robusta | XM_009013566 |
| Polychaeta | Capitellida | Capitella teleta | KB309928 |
| Phylum Gastropoda (Molluscs) | |||
| Class | Order | Species | Accession number |
| Gastropoda | Lottiida | Lottia gigantea | XM_009047767 |
| Bivalvia | Ostreoida | Crassostrea gigas | XM_011447075 |
| Phylum Platyhelminthes (flatworms) | |||
| Class | Order | Species | Accession number |
| Trematoda | Strigeidida | Schistosoma mansoni | CABG01000042.1 |
| Trematoda | Prosostomata | Schistosoma haematobium | XM_012938931.1 |
| Trematoda | Opisthorchiida | Opisthorchis viverrini | XM_009168655 |
| Trematoda | Opisthorchiida | Clonorchis sinensis | DF143054 |
| Cestoda | Cyclophyllidea | Echinococcus multilocularis | LK031422.1 |
| Cestoda | Cyclophyllidea | Echinococcus granulosus | LK028580.1 |
| Cestoda | Cyclophyllidea | Hymenolepis microstoma | LK053266.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essig, Y.J.; Webb, S.M.; Stürzenbaum, S.R. Deletion of Phytochelatin Synthase Modulates the Metal Accumulation Pattern of Cadmium Exposed C. elegans. Int. J. Mol. Sci. 2016, 17, 257. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17020257
Essig YJ, Webb SM, Stürzenbaum SR. Deletion of Phytochelatin Synthase Modulates the Metal Accumulation Pattern of Cadmium Exposed C. elegans. International Journal of Molecular Sciences. 2016; 17(2):257. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17020257
Chicago/Turabian StyleEssig, Yona J., Samuel M. Webb, and Stephen R. Stürzenbaum. 2016. "Deletion of Phytochelatin Synthase Modulates the Metal Accumulation Pattern of Cadmium Exposed C. elegans" International Journal of Molecular Sciences 17, no. 2: 257. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17020257