Inhibition of SDF-1α/CXCR4 Signalling in Subchondral Bone Attenuates Post-Traumatic Osteoarthritis
Abstract
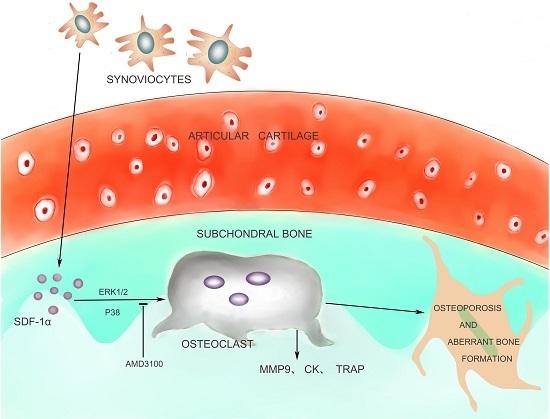
:1. Introduction
2. Results
2.1. Tibial Subchondral Bone Analysis via μCTimaging
2.2. Elevated Active SDF-1α and Bone Resorption in Subchondral Bone
2.3. Inhibition of SDF-1α Signalling in Subchondral Bone Attenuates Cartilage Degeneration
2.4. SDF-1α and CTX-I Concentrations in Serum
2.5. Effects of SDF-1α/CXCR4 Signalling on Osteoclast Differentiation in Vitro
2.6. Effects of SDF-1α/CXCR4 Signalling on Multiple Pathways Involved in Osteoclast Differentiation
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animals and Experimental Groups
4.3. Micro-Computed Tomography (μCT) Imaging
4.4. Histochemistry and Immunohistochemistry
4.5. Serum Biochemistry
4.6. Cell Cultures
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Lories, R.J.; Luyten, F.P. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Neogi, T. Osteoarthritis: Is it a disease of cartilage or of bone? Arthritis Rheum. 2004, 50, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Mansell, J.P.; Collins, C.; Bailey, A.J. Bone, not cartilage, should be the major focus in osteoarthritis. Nat. Clin. Pract. Rheumatol. 2007, 3, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Hayami, T.; Pickarski, M.; Zhuo, Y.; Wesolowski, G.A.; Rodan, G.A.; Duong, L.T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 2006, 38, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.S.; Diko, S.; Hsia, A.W.; Anderson, M.J.; Genetos, D.C.; Haudenschild, D.R.; Christiansen, B.A. Effect of alendronate on post-traumatic osteoarthritis induced by anterior cruciate ligament rupture in mice. Arthritis Res. Ther. 2015, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Bay-Jensen, A.C.; Lories, R.J.; Abramson, S.; Spector, T.; Pastoureau, P.; Christiansen, C.; Attur, M.; Henriksen, K.; Goldring, S.R.; et al. The coupling of bone and cartilage turnover in osteoarthritis: Opportunities for bone antiresorptives and anabolics as potential treatments? Ann. Rheum. Dis. 2014, 73, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Funck-Brentano, T.; Lin, H.; Ea, H.K.; Hannouche, D.; Marty, C.; Liote, F.; Geoffroy, V.; Cohen-Solal, M.E. Inhibition of bone resorption blunts osteoarthritis in mice with high bone remodelling. Ann. Rheum. Dis. 2010, 69, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Hayami, T.; Pickarski, M.; Wesolowski, G.A.; McLane, J.; Bone, A.; Destefano, J.; Rodan, G.A.; Duong, L.T. The role of subchondral bone remodeling in osteoarthritis: Reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004, 50, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, J.; Ryu, J.H.; Oh, H.; Chun, C.H.; Kim, B.J.; Min, B.H.; Chun, J.S. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Fukai, A.; Mabuchi, A.; Ikeda, T.; Yano, F.; Ohba, S.; Nishida, N.; Akune, T.; Yoshimura, N.; Nakagawa, T.; et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 2010, 16, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.P.; Li, P.; Fleming, B.C.; Chen, Q.; Wei, X.; Hua, P.X.; Li, G.; Wei, L. Attenuation of cartilage pathogenesis in post-traumatic osteoarthritis (PTOA) in mice by blocking the stromal derived factor 1 receptor (CXCR4) with the specific inhibitor, AMD3100. J. Orthop. Res. 2015, 33, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Kanbe, K.; Lee, M.; Wei, X.; Pei, M.; Sun, X.; Terek, R.; Chen, Q. Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev. Biol. 2010, 341, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Yang, R.S.; Hsieh, K.H.; Fong, Y.C.; Way, T.D.; Lee, T.S.; Wu, H.C.; Fu, W.M.; Tang, C.H. Stromal cell-derived factor-1 induces matrix metalloprotease-13 expression in human chondrocytes. Mol. Pharmacol. 2007, 72, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Mazzetti, I.; Magagnoli, G.; Paoletti, S.; Uguccioni, M.; Olivotto, E.; Vitellozzi, R.; Cattini, L.; Facchini, A.; Borzi, R.M. A role for chemokines in the induction of chondrocyte phenotype modulation. Arthritis Rheum. 2004, 50, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Kanbe, K.; Takemura, T.; Takeuchi, K.; Chen, Q.; Takagishi, K.; Inoue, K. Synovectomy reduces stromal-cell-derived factor-1 (SDF-1) which is involved in the destruction of cartilage in osteoarthritis and rheumatoid arthritis. J. Bone Jt. Surg. Br. 2004, 86, 296–300. [Google Scholar] [CrossRef]
- Kim, G.W.; Han, M.S.; Park, H.R.; Lee, E.J.; Jung, Y.K.; Usmani, S.E.; Ulici, V.; Han, S.W.; Beier, F. CXC chemokine ligand 12a enhances chondrocyte proliferation and maturation during endochondral bone formation. Osteoarthr. Cartil. 2015, 23, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Guang, L.G.; Boskey, A.L.; Zhu, W. Regulatory role of stromal cell-derived factor-1 in bone morphogenetic protein-2-induced chondrogenic differentiation in vitro. Int. J. Biochem. Cell Biol. 2012, 44, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, A.; Frank, E.; Allred, C.; Jones, E.; Chen, M.; Zhao, W.; Mao, J.J. Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J. 2011, 25, 3496–3504. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy-Thandavan, S.; Herberg, S.; Arounleut, P.; Upadhyay, S.; Dukes, A.; Davis, C.; Johnson, M.; McGee-Lawrence, M.; Hamrick, M.W.; Isales, C.M.; et al. Caloric restriction and the adipokine leptin alter the SDF-1 signaling axis in bone marrow and in bone marrow derived mesenchymal stem cells. Mol. Cell. Endocrinol. 2015, 410, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.; Cristino, S.; Toneguzzi, S.; Piacentini, A.; Facchini, A.; Lisignoli, G. CXCL12 chemokine up-regulates bone resorption and MMP-9 release by human osteoclasts: CXCL12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. J. Cell. Physiol. 2004, 199, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Chuang, J.Y.; Fong, Y.C.; Maa, M.C.; Way, T.D.; Hung, C.H. Bone-derived SDF-1 stimulates IL-6 release via CXCR4, ERK and NF-κB pathways and promotes osteoclastogenesis in human oral cancer cells. Carcinogenesis 2008, 29, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.M.; Maloney, W.; Yu, X.; Kindle, L.; Collin-Osdoby, P.; Osdoby, P. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone 2005, 36, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Im, J.Y.; Min, W.K.; Park, M.H.; Kim, N.; Lee, J.K.; Jin, H.K.; Choi, J.Y.; Kim, S.Y.; Bae, J.S. AMD3100 improves ovariectomy-induced osteoporosis in mice by facilitating mobilization of hematopoietic stem/progenitor cells. BMB Rep. 2014, 47, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Diamond, P.; Labrinidis, A.; Martin, S.K.; Farrugia, A.N.; Gronthos, S.; To, L.B.; Fujii, N.; O’Loughlin, P.D.; Evdokiou, A.; Zannettino, A.C. Targeted disruption of the CXCL12/CXCR4 axis inhibits osteolysis in a murine model of myeloma-associated bone loss. J. Bone Miner. Res. 2009, 24, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Leong, W.; Su, K.; Fang, Y.; Wang, D.A. A transduced living hyaline cartilage graft releasing transgenic stromal cell-derived factor-1 inducing endogenous stem cell homing in vivo. Tissue Eng. A 2013, 19, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, A.; Iwasaki, N.; Kasahara, Y.; Onodera, T.; Igarashi, T.; Minami, A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng. A 2012, 18, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Funck-Brentano, T.; Lin, H.; Hay, E.; Ah Kioon, M.D.; Schiltz, C.; Hannouche, D.; Nizard, R.; Liote, F.; Orcel, P.; de Vernejoul, M.C.; et al. Targeting bone alleviates osteoarthritis in osteopenic mice and modulates cartilage catabolism. PLoS ONE 2012, 7, e33543. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Moore, D.C.; Wei, L.; Li, Y.; Zhang, G.; Wei, X.; Lee, J.K.; Chen, Q. Attenuation of osteoarthritis via blockade of the SDF-1/CXCR4 signaling pathway. Arthritis Res. Ther. 2012, 14, R177. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Bigliani, L.U.; Fujisawa, M.; Murakami, K.; Chang, S.S.; Lee, H.J.; Lee, F.Y.; Blaine, T.A. Stromal cell-derived factor 1 (SDF-1, CXCL12) is increased in subacromial bursitis and downregulated by steroid and nonsteroidal anti-inflammatory agents. J. Orthop. Res. 2006, 24, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Tsou, H.K.; Hsu, C.J.; Tsai, C.H.; Kao, C.H.; Fong, Y.C.; Tang, C.H. Stromal cell-derived factor-1/CXCR4 promotes IL-6 production in human synovial fibroblasts. J. Cell. Biochem. 2011, 112, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zhao, L.; Cao, H.; Chen, A.; Xiao, J. Epoxyeicosanoids suppress osteoclastogenesis and prevent ovariectomy-induced bone loss. FASEB J. 2015, 29, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Moodie, J.P.; Stok, K.S.; Muller, R.; Vincent, T.L.; Shefelbine, S.J. Multimodal imaging demonstrates concomitant changes in bone and cartilage after destabilisation of the medial meniscus and increased joint laxity. Osteoarthr. Cartil. 2011, 19, 163–170. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. The AMD3100 story: The path to the discovery of a stem cell mobilizer (Mozobil). Biochem. Pharmacol. 2009, 77, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Pusic, I.; DiPersio, J.F. Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr. Opin. Hematol. 2010, 17, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18, S17–S23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, H.; Li, J.; Fang, Z.; Chen, W.; Li, F. Amlexanox suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss. Sci. Rep. 2015, 5, 13575. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Liu, H.; Zhang, X.; Xu, F.; Qin, L.; Cheng, P.; Huang, H.; Guo, F.; Yang, Q.; Chen, A. Inhibition of SDF-1α/CXCR4 Signalling in Subchondral Bone Attenuates Post-Traumatic Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 943. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060943
Dong Y, Liu H, Zhang X, Xu F, Qin L, Cheng P, Huang H, Guo F, Yang Q, Chen A. Inhibition of SDF-1α/CXCR4 Signalling in Subchondral Bone Attenuates Post-Traumatic Osteoarthritis. International Journal of Molecular Sciences. 2016; 17(6):943. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060943
Chicago/Turabian StyleDong, Yonghui, Hui Liu, Xuejun Zhang, Fei Xu, Liang Qin, Peng Cheng, Hui Huang, Fengjing Guo, Qing Yang, and Anmin Chen. 2016. "Inhibition of SDF-1α/CXCR4 Signalling in Subchondral Bone Attenuates Post-Traumatic Osteoarthritis" International Journal of Molecular Sciences 17, no. 6: 943. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060943