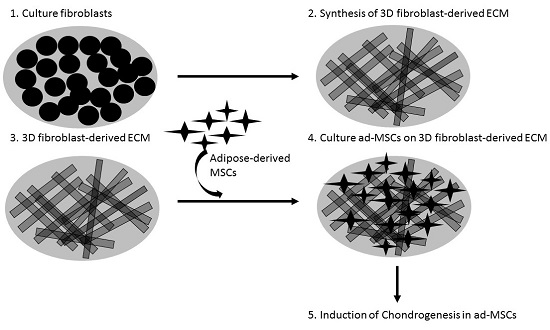
Fibroblast-Derived Extracellular Matrix Induces Chondrogenic Differentiation in Human Adipose-Derived Mesenchymal Stromal/Stem Cells in Vitro
Abstract
:1. Introduction
2. Results
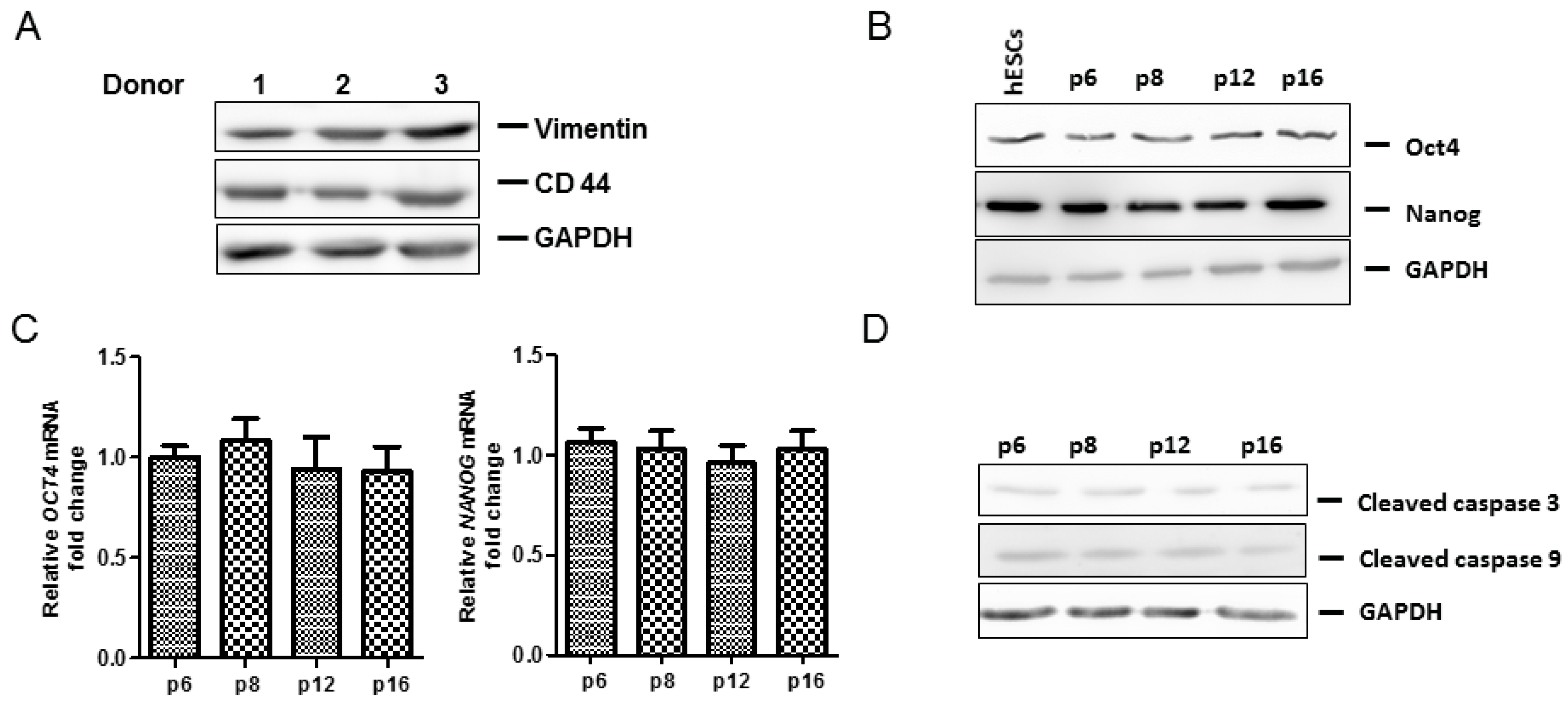
2.1. Adipose-Derived Mesenchymal Stromal/Stem Cells (MSCs) Characterization
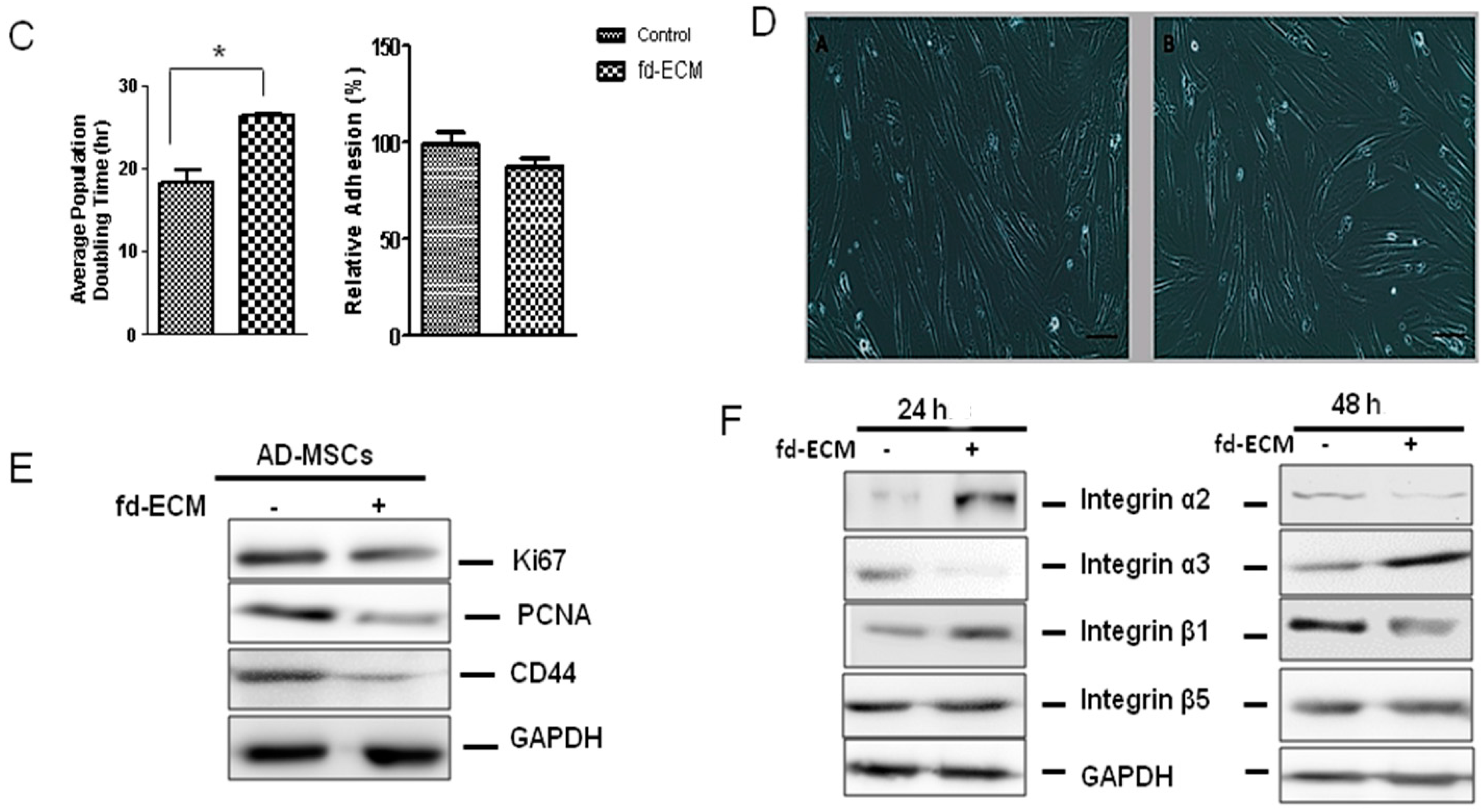
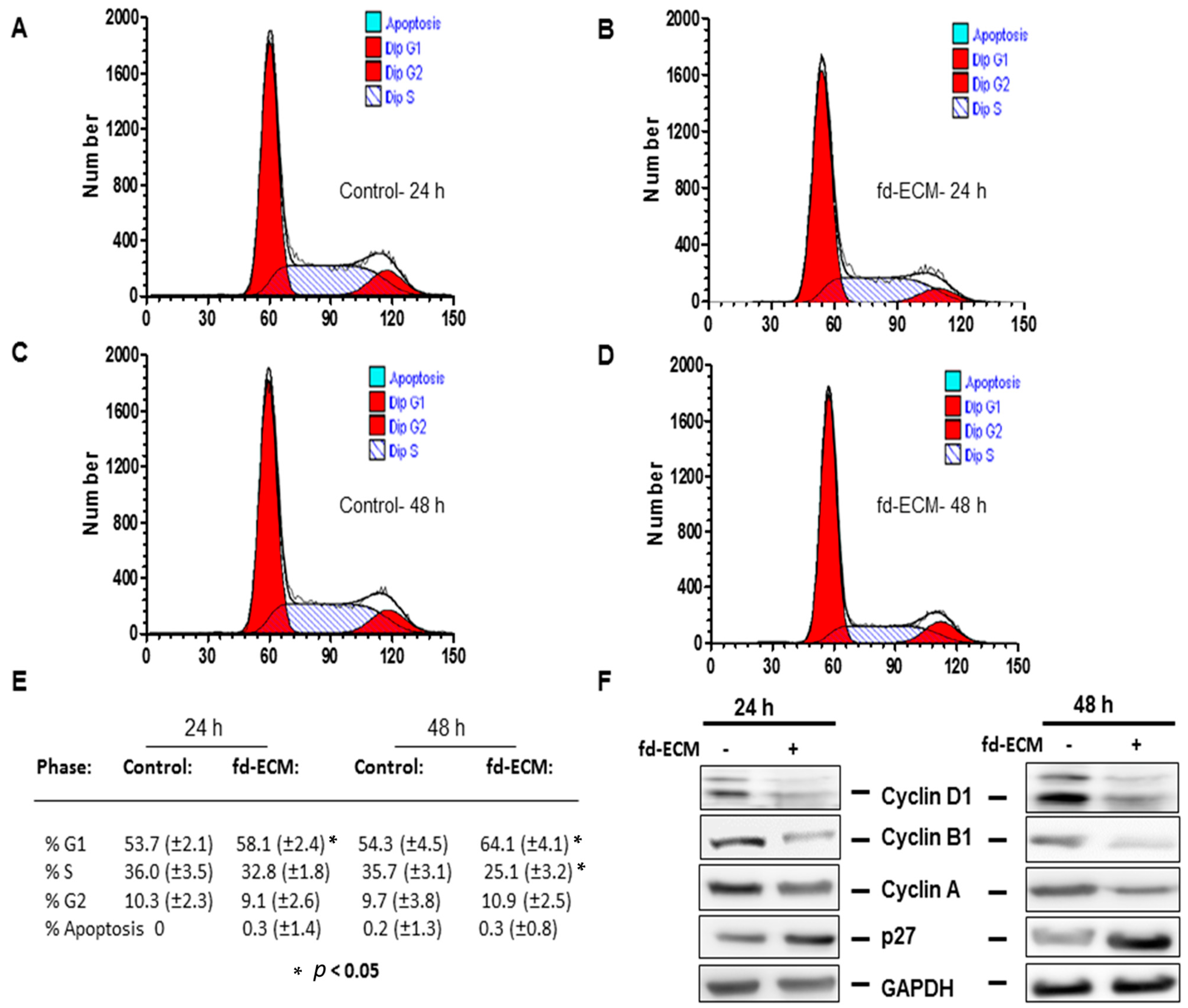
2.2. Proliferative Capacity and Morphology of Adipose-Derived MSCs (Ad-MSCs) Cultured on a Fibroblast-Derived Extracellular Matrix (Fd-ECM)
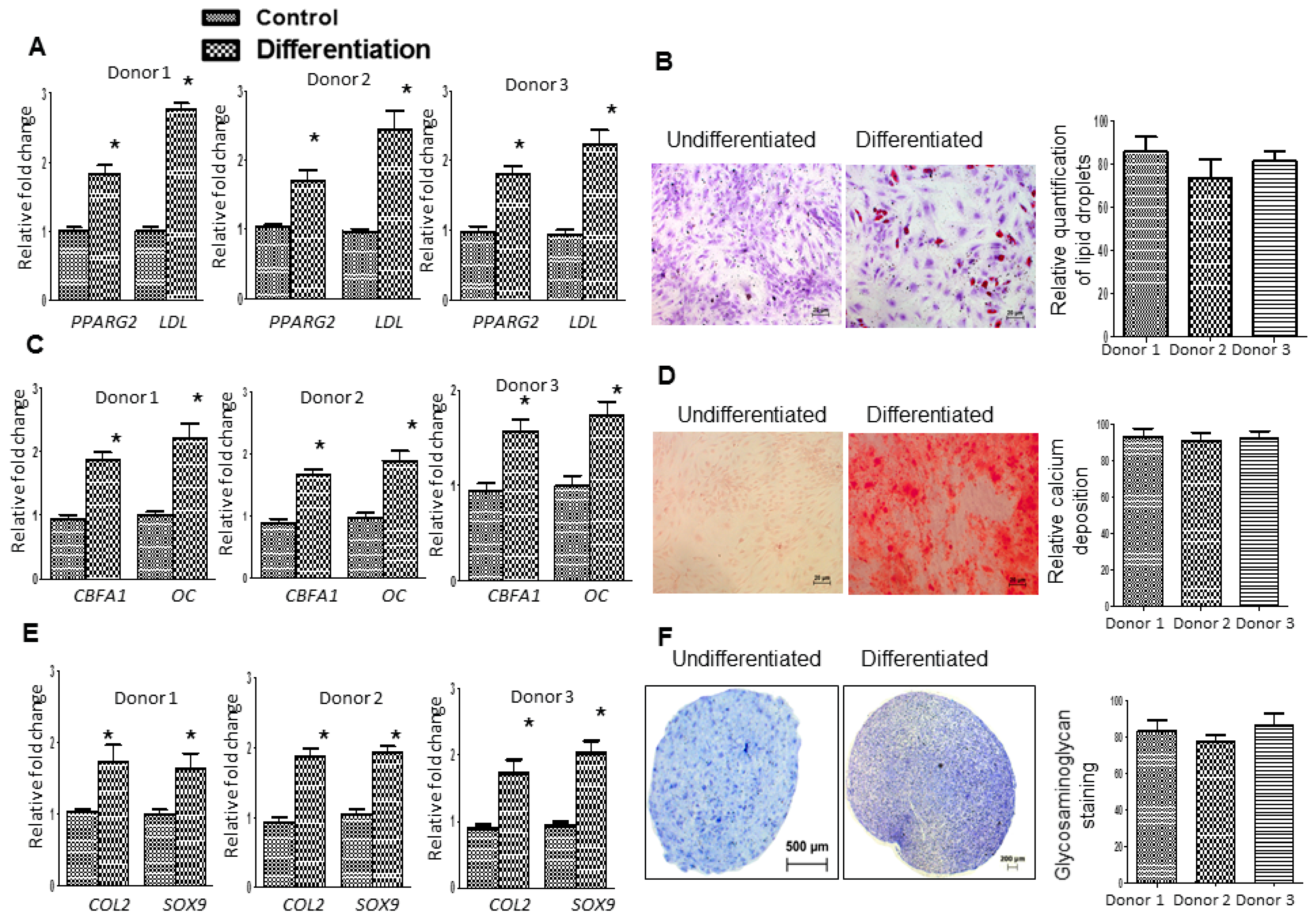
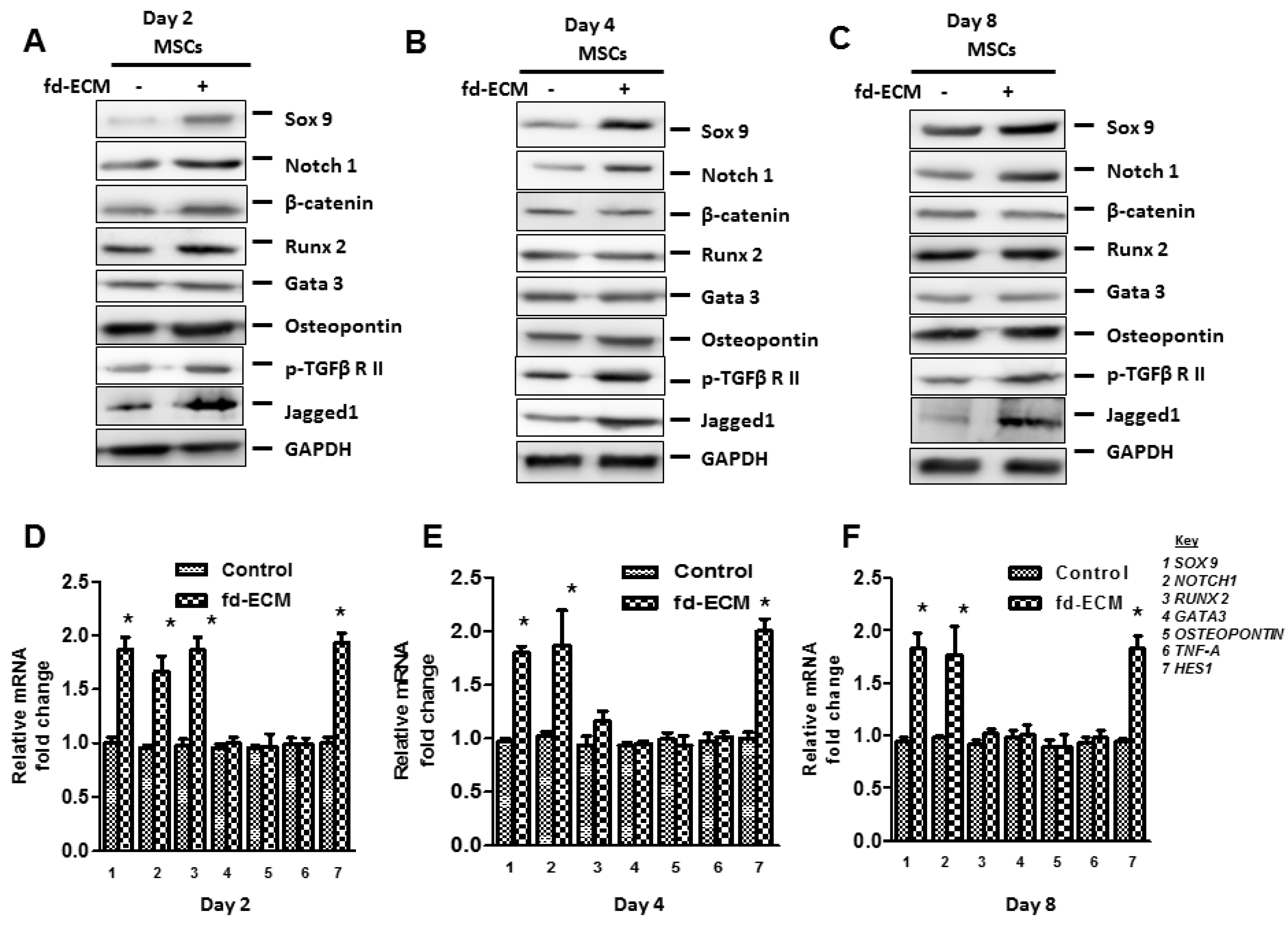
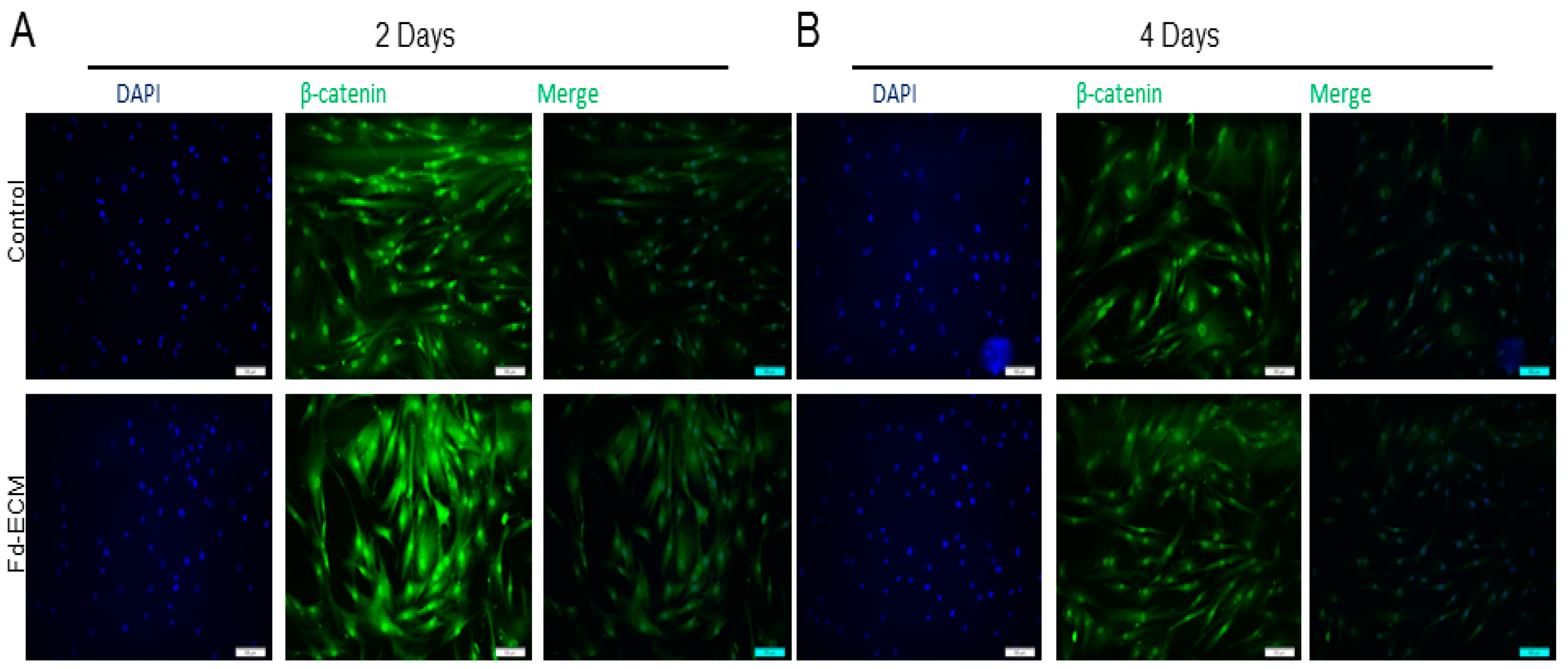
2.3. Fd-ECM Directs Ad-MSCs Differentiation towards the Chondrogenic Lineage
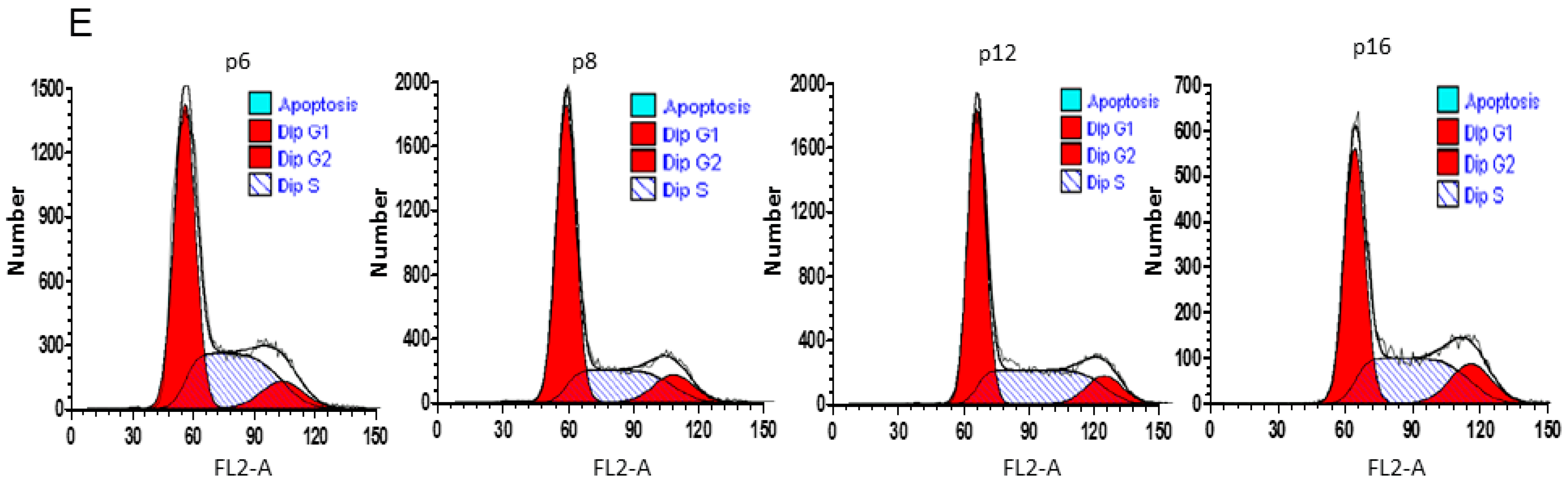
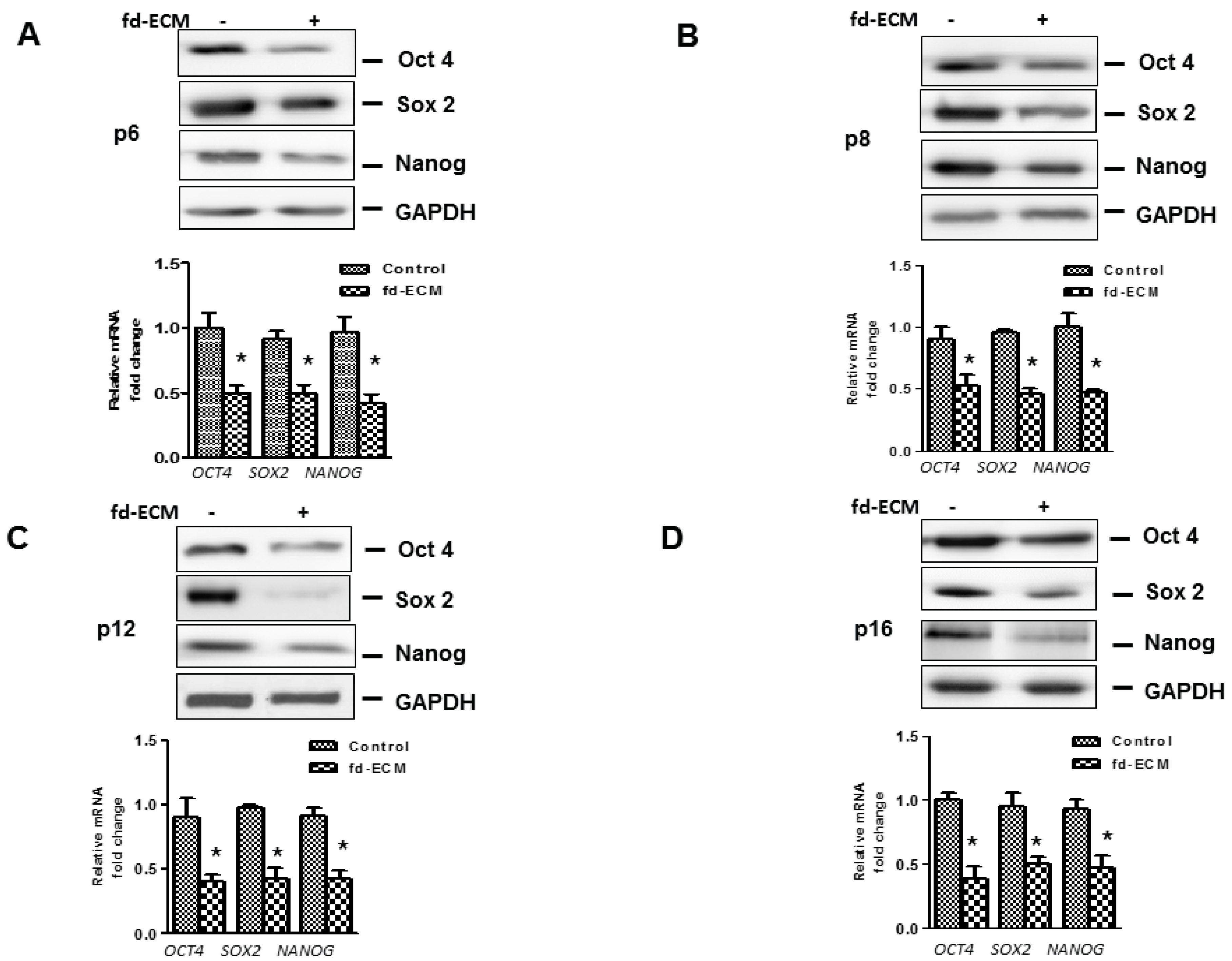
2.4. Anti-Senescence Effect of Fd-ECM on Ad-MSCs
2.5. Effect of Fd-ECM on Transformation Markers
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Fd-ECM and Cell Culture
4.3. Adipose Tissue Procurement and Processing
4.4. Immunophenotyping and Differentiation of Ad-MSCs
4.5. RNA Preparation and RT-qPCR
4.6. Cellular Adhesion Assays
4.7. Ad-MSCs Doubling Time (Gt)
4.8. Immunoblot Analysis
4.9. Cell Cycle Analysis
4.10. Transient Transfection Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bissell, M.J.; Barcellos-Hoff, M.H. The influence of extracellular matrix on gene expression: Is structure the message? J. Cell Sci. 1987, 8, 327–343. [Google Scholar] [CrossRef]
- Hay, E.D. Collagen and other matrix glycoproteins in embryogenesis. In Cell Biology of Extracellular Matrix, 2nd ed.; Hay, E.D., Ed.; Plenum Press: New York, NY, USA, 1991; pp. 419–456. [Google Scholar]
- Green, J.A.; Yamada, K.M. Three-dimensional microenvironments modulate fibroblast signaling responses. Adv. Drug Deliv. Rev. 2007, 59, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.D.; Gentleman, E.; Chen, X.; Roberts, C.J.; Polak, J.M.; Stevens, M.M. Extracellular matrix-mediated osteogenic differentiation of murine embryonic stem cells. Biomaterials 2010, 31, 3244–3252. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Leaner, V.D.; Parker, M.I. Feedback regulation of the α2(1) collagen gene via the MEK-ERK signaling pathway. IUBMB Life 2012, 64, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Leaner, V.D.; Parker, M.I. Absence of feedback regulation in the synthesis of COL1A1. Life Sci. 2014, 103, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, Y.; Bharadwaj, S.; Hammam, N.; Carnagey, K.; Myers, R.; Atala, A.; van Dyke, M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 2009, 30, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Hanagata, N.; Takemura, T.; Monkawa, A.; Ikoma, T.; Tanaka, J. Pre-adsorbed type I collagen structure-dependent changes in osteoblastic phenotype. Biochem. Biophys. Res. Commun. 2006, 344, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Oberwallner, B.; Brodarac, A.; Anic, P.; Saric, T.; Wassilew, K.; Neef, K.; Choi, Y.H.; Stamm, C. Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. Eur. J. Cardio-Thorac. Surg. 2015, 47, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Vogelsang, M.; Parker, M.I. Wnt/β-catenin and MEK-ERK signaling are required for fibroblast-derived extracellular matrix-mediated endoderm differentiation of embryonic stem cells. Stem Cell Rev. Rep. 2015, 11, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Vogelsang, M.; Thomford, N.E.; Dandara, C.; Kallmeyer, K.; Pepper, M.S.; Parker, M.I. Wharton’s Jelly-Derived mesenchymal stromal cells and fibroblast-derived extracellular matrix synergistically activate apoptosis in a p21-dependent mechanism in WHCO1 and MDA MB 231 cancer cells in vitro. Stem Cells Int. 2016, 2016, 4842134. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.J.; Robertson, E.J. Making a commitment: Cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 2009, 10, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Van Vollenstee, F.A.; Jackson, C.; Hoffmann, D.; Potgieter, M.; Durandt, C.; Pepper, M.S. Human adipose derived mesenchymal stromal cells transduced with GFP lentiviral vectors: Assessment of immunophenotype and differentiation capacity in vitro. Cytotechnology 2016. [Google Scholar] [CrossRef] [PubMed]
- Durandt, C.; van Vollenstee, F.A.; Dessels, C.; Kallmeyer, K.; de Villiers, D.; Murdoch, C.; Potgieter, M.; Pepper, M.S. Novel flow cytometric approach for the detection of adipocyte sub-populations during adipogenesis. J. Lipid Res. 2016, 57, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Robey, P.G.; Kuznetsov, S.A.; Ren, J.; Klein, H.G.; Sabatino, M.; Stroncek, D.F. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone 2015, 70, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Wilschut, K.J.; Haagsmanb, H.P.; Roelena, B.A.J. Extracellular matrix components direct porcine muscle stem cell behavior. Exp. Cell Res. 2010, 316, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Scannell, D.R.; Eisen, M.B.; Tjian, R. Control of embryonic stem cell lineage commitment by core promoter factor, TAF3. Cell 2011, 146, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Irwin, E.; Gupta, R.; Dashti, D.; Healy, K.E. Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials 2011, 32, 6912–6919. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lu, C.; Meng, D.; Xiao, Z.; Hou, X.; Ding, W.; Kou, D.; Yao, Y.; Chen, B.; Zhang, Z.; et al. Collagen scaffolds modified with CNTF and BFGF promote facial nerve regeneration in minipigs. Biomaterials 2014, 35, 7819–7827. [Google Scholar] [CrossRef] [PubMed]
- Serebriiskii, I.; Castelló-Cros, R.; Lamb, A.; Golemis, E.A.; Cukierman, E. Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol. 2008, 27, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, D.; Oberdoerfer, D.; Fernandes, M.C.; Meurer, R.T.; Pereira-Filho, G.A.; Cruz, P.; Vargas, M.; Chem, R.C.; Camassola, M.; Nardi, N.B. Mesenchymal stem cells combined with an artificial dermal substitute improve repair in full-thickness skin wounds. Burns 2012, 38, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Zhang, Y.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.J.; Grande, D.A.; Dines, J.S. The use of mesenchymal stem cells in tissue engineering: A global assessment. Organogenesis 2008, 4, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.M.; Gibran, N.S. Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp. Cell Res. 2010, 316, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.; Pisani, D.; Dechesne, C.A.; Turc-Carel, C.; Kurzenne, J.Y.; Wdziekonski, B.; Villageois, A.; Bagnis, C.; Breittmayer, J.P.; Groux, H.; et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J. Exp. Med. 2005, 201, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Pommey, S.; Eliopoulos, N.; Galipeau, J. Interferon-γ-stimulated mar-row stromal cells: A new type of nonhematopoietic antigen-presenting cell. Blood 2006, 107, 2570–2577. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, F.; Azzollini, N.; Cassis, P.; Imberti, B.; Morigi, M.; Cuqini, D.; Cavinato, R.A.; Todeschini, M.; Solini, S.; Sonzoqni, A.; et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semi allogeneic heart transplant through the generation of regulatory T cells. J. Immunol. 2008, 181, 3933–3946. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.; Guilak, F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 2003, 5, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Meyerrose, T.E.; de Ugarte, D.A.; Hofling, A.A.; Herrbrich, P.E.; Cordonnier, T.D.; Schultz, L.D.; Eagon, J.C.; Wirthlin, L.; Sands, M.S.; Hedrick, M.A.; et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells 2007, 25, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 2010, 31, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.J.; Blaschke, A.J.; Buchholz, D.E.; Hikita, S.T.; Johnson, L.V.; Clegg, D.O. Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. J. Tissue Eng. Regen. Med. 2013, 7, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; de Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.L.; Wang, B.; Squire, M.W.; Guo, L.W.; Li, W.J. Endothelial cells direct human mesenchymal stem cells for osteo-and chondro-lineage differentiation through endothelin-1 and AKT signaling. Stem Cell Res. Ther. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Canela, A.; Vera, E.; Tejera, A.; Cotsarelis, G.; Blasco, M.A. The longest telomeres: A general signature of adult stem cell compartments. Genes Dev. 2008, 22, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Donate, L.E.; Blasco, M.A. Telomeres in cancer and ageing. Philos. Trans. R. Soc. B: Biol. Sci. 2011, 366, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yuan, Y.; Chi, F. Biomimetic alginate/polyacrylamide porous scaffold supports human mesenchymal stem cell proliferation and chondrogenesis. Mater. Sci. Eng: C 2014, 42, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Li, P.; de Crombrugghe, B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are co-expressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998, 17, 5718–5733. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; de Crombrugghe, B. Toward understanding Sox9 function in chondrocyte differentiation. Matrix Biol. 1998, 16, 529–540. [Google Scholar] [CrossRef]
- Akiyama, H.; Lefebvre, V. Unravelling the transcriptional regulatory machinery in chondrogenesis. J. Bone Miner Metab. 2011, 29, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.; Dong, Y.; Mirando, A.J.; Jesse, A.M.; Honjo, T.; Zuscik, M.J.; O’Keefe, R.J.; Hilton, M.J. Cartilage-specific RBPjκ-dependent and independent Notch signals regulate cartilage and bone development. Development 2012, 139, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.M.; Czepiel, M.; Cetinkaya, C.; Smigielska, K.; Weli, S.C.; Lysdahl, H.; Gabrielsen, A.; Petersen, K.; Ehlers, N.; Fink, T.; et al. Continuous hypoxic culturing maintains activation of Notch and allows long-term propagation of human embryonic stem cells without spontaneous differentiation. Cell Prolif. 2009, 42, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Nekanti, U.; Dastidar, S.; Venugopal, P.; Totey, S.; Ta, M. Increased proliferation and analysis of differential gene expression in human Wharton’s jelly-derived mesenchymal stromal cells under hypoxia. Int. J. Biol. Sci. 2010, 6, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Heeschen, C.; Lehmann, R.; Honold, J.; Assmus, B.; Aicher, A.; Walter, D.H.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 2004, 109, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Jeong, S.G.; Cho, G.W. Anti-senescence effects of DNA methyltransferase inhibitor RG108 in human bone marrow mesenchymal stromal cells. Biotechnol. Appl. Biochem. 2015, 62, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kurtz, A.; Stamm, C. Mesenchymal stem cells for cardiac cell therapy. Hum. Gene Ther. 2010, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Serakinci, N.; Graakjaer, J.; Kolvraa, S. Telomere stability and telomerase in mesenchymal stem cells. Biochimie 2008, 90, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E. Preparation of extracellular matrices produced by cultured fibroblasts. In Current Protocols in Cell Biology; Bonifacino, J.S., Dasso, M., Lippincott-Schwartz, J., Harford, J.B., Yamada, K.M., Eds.; John Wiley and Sons: New York, NY, USA, 2002; pp. 10.09.01–10.09.14. [Google Scholar] [CrossRef]
- Cukierman, E. Cell migration analyses within fibroblast-derived 3-D matrices. In Cell Migration: Developmental Methods and Protocols; Guan, J., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 70–93. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1983, 132, 6–13. [Google Scholar] [CrossRef]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzobo, K.; Turnley, T.; Wishart, A.; Rowe, A.; Kallmeyer, K.; Van Vollenstee, F.A.; Thomford, N.E.; Dandara, C.; Chopera, D.; Pepper, M.S.; et al. Fibroblast-Derived Extracellular Matrix Induces Chondrogenic Differentiation in Human Adipose-Derived Mesenchymal Stromal/Stem Cells in Vitro. Int. J. Mol. Sci. 2016, 17, 1259. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081259
Dzobo K, Turnley T, Wishart A, Rowe A, Kallmeyer K, Van Vollenstee FA, Thomford NE, Dandara C, Chopera D, Pepper MS, et al. Fibroblast-Derived Extracellular Matrix Induces Chondrogenic Differentiation in Human Adipose-Derived Mesenchymal Stromal/Stem Cells in Vitro. International Journal of Molecular Sciences. 2016; 17(8):1259. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081259
Chicago/Turabian StyleDzobo, Kevin, Taegyn Turnley, Andrew Wishart, Arielle Rowe, Karlien Kallmeyer, Fiona A. Van Vollenstee, Nicholas E. Thomford, Collet Dandara, Denis Chopera, Michael S. Pepper, and et al. 2016. "Fibroblast-Derived Extracellular Matrix Induces Chondrogenic Differentiation in Human Adipose-Derived Mesenchymal Stromal/Stem Cells in Vitro" International Journal of Molecular Sciences 17, no. 8: 1259. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081259