Sugarcane Serine Peptidase Inhibitors, Serine Peptidases, and Clp Protease System Subunits Associated with Sugarcane Borer (Diatraea saccharalis) Herbivory and Wounding
Abstract
:1. Introduction
2. Results
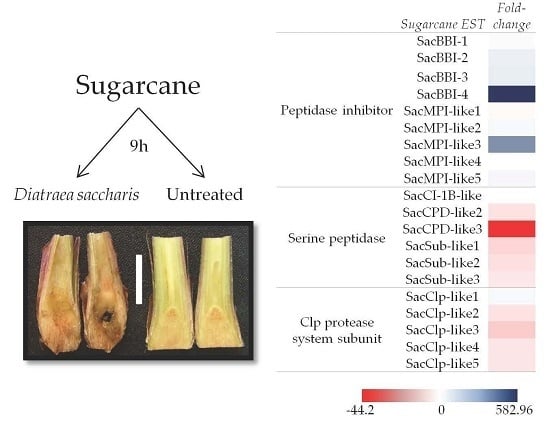
2.1. Macroarray Hybridization
2.2. Validation of D. saccharalis-Inducible Genes
2.2.1. Sugarcane Bowman-Birk Inhibitor (SacBBI) Genes
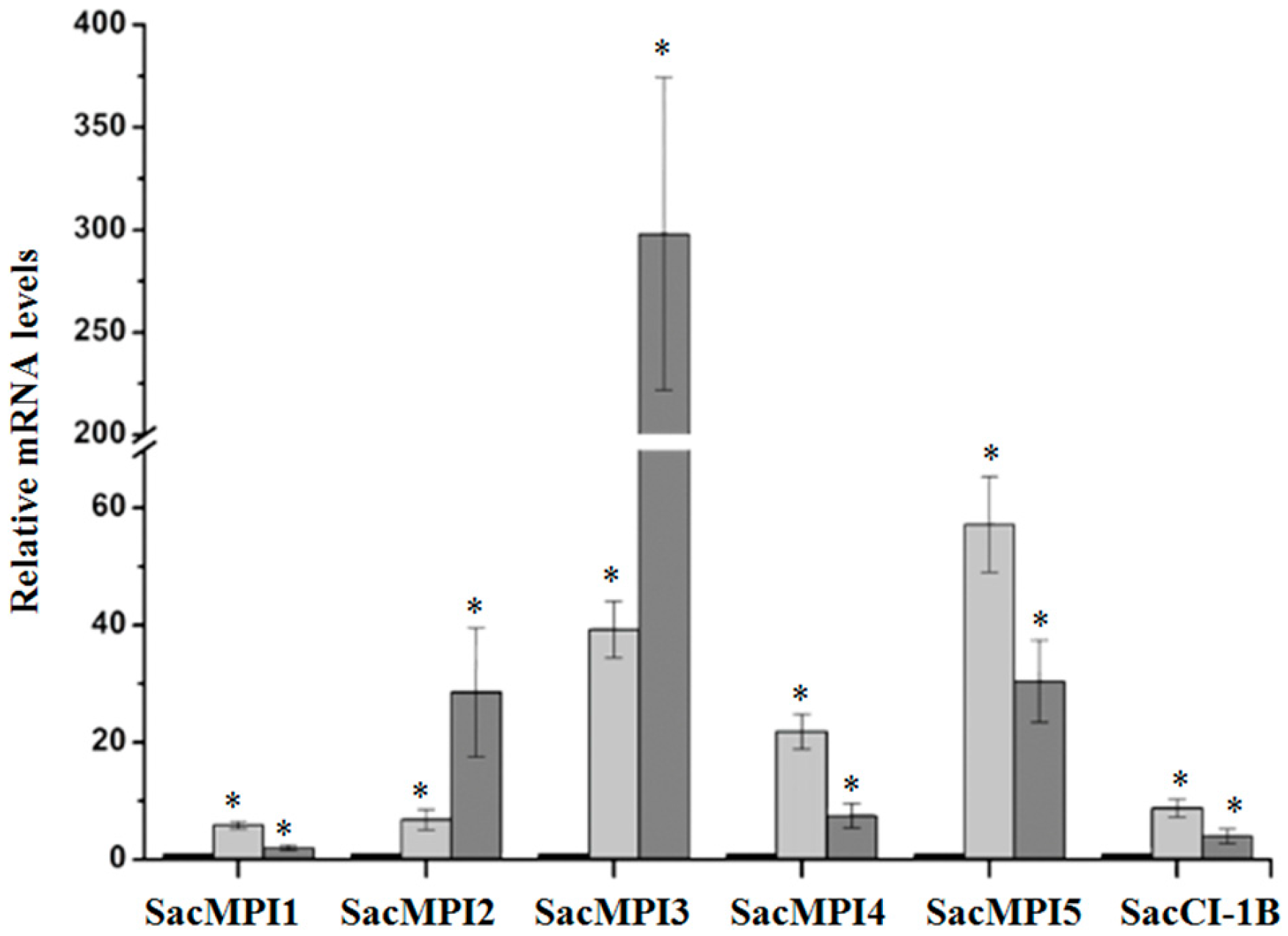
2.2.2. Sugarcane Maize-peptidase-inhibitor-like (SacMPI-like) and Chymotrypsin Inhibitor 1B-like (SacCI1B-like) Genes
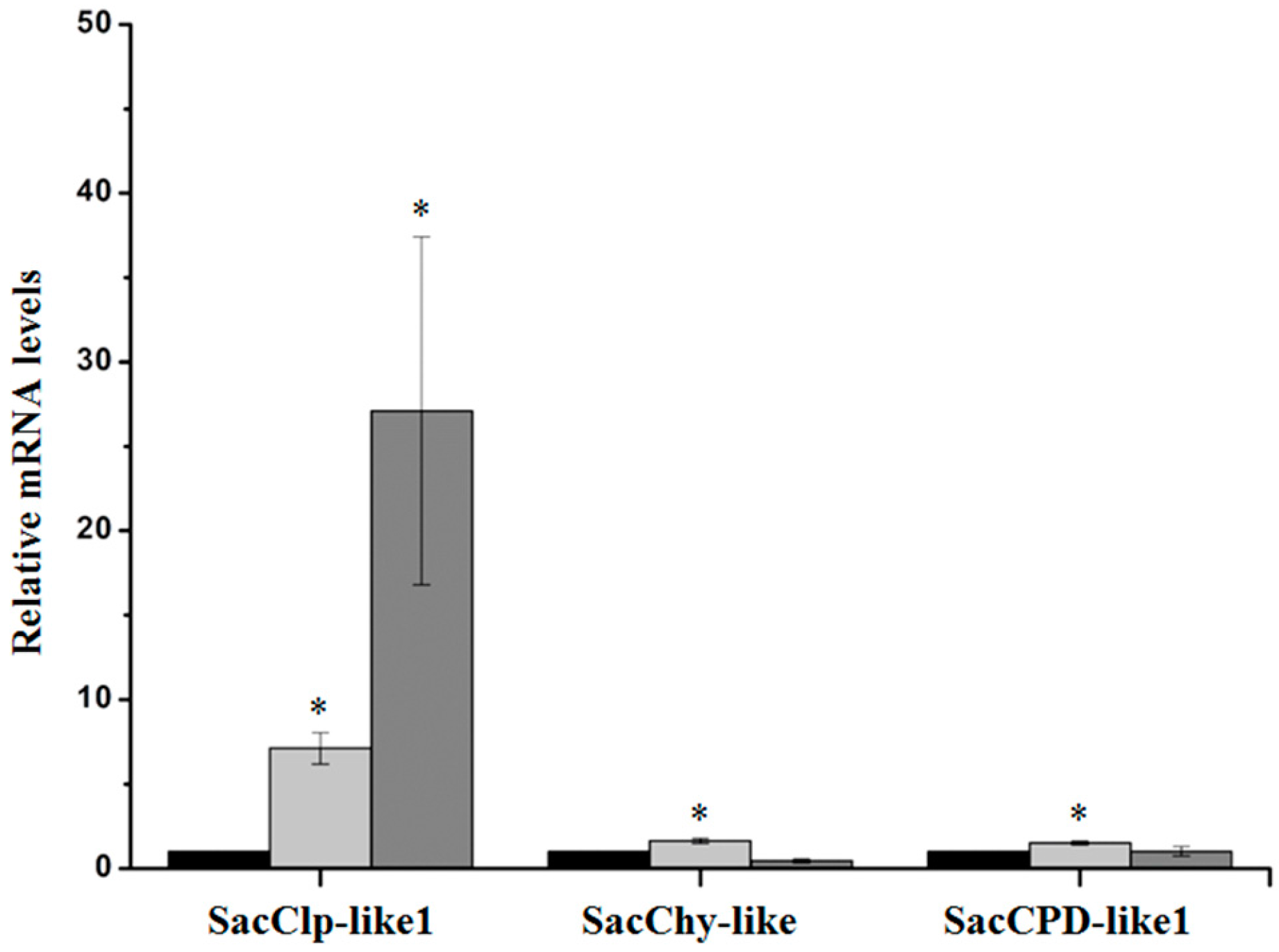
2.2.3. Serine Peptidases and Clp Protease System Subunits
2.3. Validation of D. saccharalis-Repressed Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material and Insects
4.1.1. Phase 1—Sugarcane and D. saccharalis Experiments Used for Macroarray Hibridization
4.1.2. Phase 2—qPCR Monitoring of Selected ESTs Identified through Macroarray
4.2. Macroarray Construction
4.3. Macroarray Normalization
4.4. Probe Preparation
4.5. Macroarray Analysis
4.6. Expression Analysis by Real-Time Quantitative PCR
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vendramim, J.; da Silva, F.; César, M.; de Camargo, A. Comparação entre dois métodos para avaliação da infestação pelo complexo broca-podridões em cultivares de cana-de-açúcar. Anais da Escola Superior de Agricultura Luiz de Queiroz 1988, 45, 397–421. [Google Scholar] [CrossRef]
- Ogunwolu, E.O.; Reagan, T.E.; Flynn, J.L.; Hensley, S.D. Effects of Diatraea saccharalis (F.) (Lepidoptera: Pyralidae) damage and stalk rot fungi on sugarcane yield in louisiana. Crop Prot. 1991, 10, 57–61. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Carroll, M.J.; LeClere, S.; Phipps, S.M.; Meredith, J.; Chourey, P.S.; Alborn, H.T.; Teal, P.E.A. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. USA 2006, 103, 8894–8899. [Google Scholar] [CrossRef] [PubMed]
- Mattiacci, L.; Dicke, M.; Posthumus, M.A. Beta-glucosidase: An elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Jones, J.D.G. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Boyko, E.V. The molecular bases of plant resistance and defense responses to aphid feeding: Current status. Entomol. Exp. Appl. 2007, 122, 1–16. [Google Scholar] [CrossRef]
- Mithofer, A.; Boland, W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 2008, 146, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.W.; Tumlinson, J.H. Plant-insect dialogs: Complex interactions at the plant-insect interface. Curr. Opin. Plant Biol. 2008, 11, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.; Ryan, C.A. Wound-induced proteinase inhibitor in plant leaves: A possible defense mechanism against insects. Science 1972, 175, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Hartl, M.; Giri, A.P.; Kaur, H.; Baldwin, I.T. The multiple functions of plant serine protease inhibitors: Defense against herbivores and beyond. Plant Signal. Behav. 2011, 6, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.A.; Beekwilder, J. Co-evolution of insect proteases and plant protease inhibitors. Curr. Protein Pept. Sci. 2011, 12, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.A. Protease inhibitors in plants: Genes for improving defenses against insects and pathogens. Annu. Rev. Phytopathol. 1990, 28, 425–449. [Google Scholar] [CrossRef]
- Bowman, D.E. Differentiation of soy bean antitryptic factors. Proc. Soc. Exp. Biol. Med. 1946, 63, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Birk, Y.; Gertler, A.; Khalef, S. A pure trypsin inhibitor from soya beans. Biochem. J. 1963, 87, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Norioka, S.; Ikenaka, T. Amino acid sequence of a trypsin-chymotrypsin inhibitor, B-III, of peanut (Arachis Hypogaea). J. Biochem. 1983, 93, 479–485. [Google Scholar] [PubMed]
- Tanaka, A.S.; Sampaio, M.U.; Marangoni, S.; deOliveira, B.; Novello, J.C.; Oliva, M.L.V.; Fink, E.; Sampaio, C.A.M. Purification and primary structure determination of a bowman-birk trypsin inhibitor from Torresea Cearensis seeds. Biol. Chem. 1997, 378, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Odani, S.; Koide, T.; Teruo, O. Wheat germ trypsin inhibitors. Isolation and structural characterization of single-headed and double-headed inhibitors of the bowman-birk type. J. Biochem. 1986, 100, 975–983. [Google Scholar] [PubMed]
- Mello, M.O.; Tanaka, A.S.; Silva-Filho, M.C. Molecular evolution of bowman-birk type proteinase inhibitors in flowering plants. Mol. Phylogenet. Evol. 2003, 27, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes—Properties, compartmentalization and function. Comp. Biochem. Physiol. Part B 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Botella, M.A.; Xu, Y.; Prabha, T.N.; Zhao, Y.; Narasimhan, M.L.; Wilson, K.A.; Nielsen, S.S.; Bressan, R.A.; Hasegawa, P.M. Differential expression of soybean cysteine proteinase inhibitor genes during development and in response to wounding and methyl jasmonate. Plant Physiol. 1996, 112, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Reymond, P.; Weber, H.; Damond, M.; Farmer, E.E. Differential gene expression in response to mechanical wounding and insect feeding in arabidopsis. Plant Cell 2000, 12, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Little, D.; Gouhier-Darimont, C.; Bruessow, F.; Reymond, P. Oviposition by pierid butterflies triggers defense responses in arabidopsis. Plant Physiol. 2007, 143, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoorn, R.A.L.; Jones, J.D. The plant proteolytic machinery and its role in defence. Curr. Opin. Plant Biol. 2004, 7, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.S.; Bergey, D.R.; Ryan, C.A. Characterization and localization of a wound-inducible type i serine-carboxypeptidase from leaves of tomato plants (Lycopersicon esculentum Mill.). Planta 2001, 212, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Tornero, P.; Conejero, V.; Vera, P. Primary structure and expression of a pathogen-induced protease (PR-P69) in tomato plants: Similarity of functional domains to subtilisin-like endoproteases. Proc. Natl. Acad. Sci. USA 1996, 93, 6332–6337. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Patankar, A.G.; Zavala, J.A.; Wu, J.Q.; Doleckova-Maresova, L.; Vujtechova, M.; Mares, M.; Baldwin, I.T. Differential elicitation of two processing proteases controls the processing pattern of the trypsin proteinase inhibitor precursor in nicotiana attenuata. Plant Physiol. 2005, 139, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.; Ryan, C.A. Identification of a 50-kDa systemin-binding protein in tomato plasma-membranes having Kex2p-like properties. Proc. Natl. Acad. Sci. USA 1994, 91, 11802–11806. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Strydom, D.; Johnson, S.; Ryan, C.A. A polypeptide from tomato leaves induces wound-inducible proteinase-inhibitor proteins. Science 1991, 253, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.A.; Moura, D.S. Systemic wound signaling in plants: A new perception. Proc. Natl. Acad. Sci. USA 2002, 99, 6519–6520. [Google Scholar] [CrossRef] [PubMed]
- Pechan, T.; Cohen, A.; Williams, W.P.; Luthe, D.S. Insect feeding mobilizes a unique plant defense protease that disrupts the peritrophic matrix of caterpillars. Proc. Natl. Acad. Sci. USA 2002, 99, 13319–13323. [Google Scholar] [CrossRef] [PubMed]
- Pechan, T.; Ye, L.; Chang, Y.-m.; Mitra, A.; Lin, L.; Davis, F.M.; Williams, W.P.; Luthe, D.S. A unique 33-kD cysteine proteinase accumulates in response to larval feeding in maize genotypes resistant to fall armyworm and other lepidoptera. Plant Cell 2000, 12, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Ma, P.W.K.; Pechan, T.; Bassford, E.R.; Williams, W.P.; Luthe, D.S. Degradation of the S. frugiperda peritrophic matrix by an inducible maize cysteine protease. J. Insect. Physiol. 2006, 52, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pautot, V.; Holzer, F.M.; Chaufaux, J.; Walling, L.L. The induction of tomato leucine aminopeptidase genes (LapA) after Pseudomonas syringae pv. tomato infection is primarily a wound response triggered by coronatine. Mol. Plant Microbe Interact. 2001, 14, 214–224. [Google Scholar] [PubMed]
- Chao, W.S.; Gu, Y.Q.; Pautot, V.; Bray, E.A.; Walling, L.L. Leucine aminopeptidase rnas, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol. 1999, 120, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.H.; Narváez-Vásquez, J.; Aromdee, D.N.; Pautot, V.; Holzer, F.M.; Walling, L.L. Leucine aminopeptidase regulates defense and wound signaling in tomato downstream of jasmonic acid. Plant Cell 2009, 21, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wilkerson, C.G.; Kuchar, J.A.; Phinney, B.S.; Howe, G.A. Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. USA 2005, 102, 19237–19242. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Vigil, E.; Bianchetti, C.M.; Phillips, G.N.; Howe, G.A. Adaptive evolution of threonine deaminase in plant defense against insect herbivores. Proc. Natl. Acad. Sci. USA 2011, 108, 5897–5902. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Jain, R.; Solomon, S.; Shrivastava, S.; Roy, A.K. Exploiting EST databases for the development and characterisation of 3425 gene-tagged CISP markers in biofuel crop sugarcane and their transferability in cereals and orphan tropical grasses. BMC Res. Notes 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Vettore, A.L.; da Silva, F.R.; Kemper, E.L.; Arruda, P. The libraries that made sucest. Genet. Mol. Biol. 2001, 24, 1–7. [Google Scholar] [CrossRef]
- Vettore, A.L.; da Silva, F.R.; Kemper, E.L.; Souza, G.M.; da Silva, A.M.; Ferro, M.I.T.; Henrique-Silva, F.; Giglioti, E.A.; Lemos, M.V.F.; Coutinho, L.L.; et al. Analysis and functional annotation of an expressed sequence tag collection for tropical crop sugarcane. Genome Res. 2003, 13, 2725–2735. [Google Scholar] [CrossRef] [PubMed]
- Casu, R.; Dimmock, C.; Thomas, M.; Bower, N.; Knight, D.; Grof, C.; McIntyre, L.; Jackson, P.; Jordan, D.; Whan, V.; et al. Genetic and expression profiling in sugarcane. Proc. Int. Soc. Sugar Cane Technol. 2001, 24, 542–546. [Google Scholar]
- Carson, D.L.; Huckett, B.I.; Botha, F.C. Sugarcane ests differentially expressed in immature and maturing internodal tissue. Plant Sci. 2002, 162, 289–300. [Google Scholar] [CrossRef]
- Ma, H.M.; Schulze, S.; Lee, S.; Yang, M.; Mirkov, E.; Irvine, J.; Moore, P.; Paterson, A. An est survey of the sugarcane transcriptome. Theor. Appl. Genet. 2004, 108, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Menossi, M.; Silva-Filho, M.C.; Vincentz, M.; Van-Sluys, M.A.; Souza, G.M. Sugarcane functional genomics: Gene discovery for agronomic trait development. Int. J. Plant Genom. 2008, 2008, 458732. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.M.; Simoes, A.C.Q.; Oliveira, K.C.; Garay, H.M.; Fiorini, L.C.; Gomes, F.D.; Nishiyama-Junior, M.Y.; da Silva, A.M. The sugarcane signal transduction (SUCAST) catalogue: Prospecting signal transduction in sugarcane. Genet. Mol. Biol. 2001, 24, 25–34. [Google Scholar] [CrossRef]
- Falco, M.C.; Marbach, P.A.S.; Pompermayer, P.; Lopes, F.C.C.; Silva-Filho, M.C. Mechanisms of sugarcane response to herbivory. Genet. Mol. Biol. 2001, 24, 113–122. [Google Scholar] [CrossRef]
- Medeiros, A.H.; Franco, F.P.; Matos, J.L.; de Castro, P.A.; Santos-Silva, L.K.; Henrique-Silva, F.; Goldman, G.H.; Moura, D.S.; Silva-Filho, M.C. Sugarwin: A sugarcane insect-induced gene with antipathogenic activity. Mol. Plant Microbe Interact. 2012, 25, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.R.; Papini-Terzi, F.S.; Nishiyama, M.Y., Jr.; Vencio, R.Z.N.; Vicentini, R.; Duarte, R.D.C.; de Rosa, V.E., Jr.; Vinagre, F.; Barsalobres, C.; Medeiros, A.H.; et al. Signal transduction-related responses to phytohormones and environmental challenges in sugarcane. BMC Genom. 2007, 8, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Brown, W.E.; Graham, J.S.; Pearce, G.; Fox, E.A.; Dreher, T.W.; Ahern, K.G.; Pearson, G.D.; Ryan, C. Molecular characterization and phylogenetic studies of a wound-inducible proteinase inhibitor i gene in lycopersicon species. Proc. Natl. Acad. Sci. USA 1986, 83, 7277–7281. [Google Scholar] [CrossRef] [PubMed]
- Bricchi, I.; Leitner, M.; Foti, M.; Mithofer, A.; Boland, W.; Maffei, M.E. Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: Early signaling and volatile emission in lima bean (Phaseolus lunatus L.). Planta 2010, 232, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Lightner, J.; Browse, J.; Ryan, C.A. An octadecanoid pathway mutant (jl5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 1996, 8, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.T.; Turlings, T.C.J.; Jones, T.H.; Stenhagen, G.; Loughrin, J.H.; Tumlinson, J.H. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acid Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, N.N.; Brover, V.V.; Freidin, S.; Troukhan, M.E.; Tatarinova, T.V.; Zhang, H.; Swaller, T.J.; Lu, Y.-P.; Bouck, J.; Flavell, R.B. Insights into corn genes derived from large-scale cdna sequencing. Plant Mol. Biol. 2009, 69, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A. The b73 maize genome: Complexity, diversity, and dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.J.; Raventos, D.; San Segundo, B. Expression of a maize proteinase inhibitor gene is induced in response to wounding and fungal infection: Systemic wound-response of a monocot gene. Plant J. 1994, 6, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, M.C.; Rufat, M.; Bravo, J.M.; San Segundo, B. Accumulation of a maize proteinase inhibitor in response to wounding and insect feeding, and characterization of its activity toward digestive proteinases of spodoptera littoralis larvae. Planta 2000, 211, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, G.; Senear, D.F.; Zuroske, G.; Ryan, C.A. Proteinase inhibitors I and II from leaves of wounded tomato plants: Purification and properties. Arch. Biochem. Biophys. 1982, 213, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; McDonald, G.; Christeller, J.T.; Lee, M.; Bateman, K.; West, J.; Van Heeswijck, R.; Anderson, M.A. Proteinase inhibitors from Nicotiana alata enhance plant resistance to insect pests. J. Insect Physiol. 1997, 43, 833–842. [Google Scholar] [CrossRef]
- Farag, M.A.; Fokar, M.; Abd, H.; Zhang, H.; Allen, R.D.; Pare, P.W. (Z)-3-hexenol induces defense genes and downstream metabolites in maize. Planta 2005, 220, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Wing, R.A.; Wise, R.P. Genome dynamics and evolution of the Mla (powdery mildew) resistance locus in barley. Plant Cell 2002, 14, 1903–1917. [Google Scholar] [CrossRef] [PubMed]
- Beßer, K.; Jarosch, B.; Langen, G.; Kogel, K.H. Expression analysis of genes induced in barley after chemical activation reveals distinct disease resistance pathways. Mol. Plant Pathol. 2000, 1, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Falco, M.C.; Silva-Filho, M.C. Expression of soybean proteinase inhibitors in transgenic sugarcane plants: Effects on natural defense against diatraea saccharalis. Plant Physiol. Biochem. 2003, 41, 761–766. [Google Scholar] [CrossRef]
- Pompermayer, P.; Lopes, A.R.; Terra, W.R.; Parra, J.R.P.; Falco, M.C.; Silva-Filho, M.C. Effects of soybean proteinase inhibitor on development, survival and reproductive potential of the sugarcane borer, diatraea saccharalis. Entomol. Exp. Appl. 2001, 99, 79–85. [Google Scholar] [CrossRef]
- Pompermayer, P.; Falco, M.C.; Parra, J.R.P.; Silva-Filho, M.C. Coupling diet quality and bowman-birk and kunitz-type soybean proteinase inhibitor effectiveness to Diatraea saccharalis development and mortality. Entomol. Exp. Appl. 2003, 109, 217–224. [Google Scholar] [CrossRef]
- Zybailov, B.; Friso, G.; Kim, J.; Rudella, A.; Rodriguez, V.R.; Asakura, Y.; Sun, Q.; van Wijk, K.J. Large scale comparative proteomics of a chloroplast clp protease mutant reveals folding stress, altered protein homeostasis, and feedback regulation of metabolism. Mol. Cell Proteom. 2009, 8, 1789–1810. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Halperin, T.; Hruskova-Heidingsfeldova, O.; Adam, Z.; Clarke, A.K. Characterization of chloroplast clp proteins in arabidopsis: Localization, tissue specificity and stress responses. Physiol. Plant 2002, 114, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Porankiewicz, J.; Schelin, J.; Clarke, A.K. The ATP-dependent Clp protease is essential for acclimation to UV-B and low temperature in the cyanobacterium Synechococcus. Mol. Microbiol. 1998, 29, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Virgos, A.; Olivella, E.; Villanueva, J.; Aviles, X.; Gabarra, R.; Prat, S. Multiple insect resistance in transgenic tomato plants over-expressing two families of plant proteinase inhibitors. Plant Mol. Biol. 2005, 57, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Maheswaran, G.; Pridmore, L.; Franz, P.; Anderson, M.A. A proteinase inhibitor from Nicotiana alata inhibits the normal development of light-brown apple moth, Epiphyas postvittana in transgenic apple plants. Plant Cell Rep. 2007, 26, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Rose, D. Microfluidic technologies and instrumentation for printing DNA microarrays. In Microarray Biochip Technology; Schena, M., Ed.; Eaton: Natic, MA, USA, 2000; pp. 19–37. [Google Scholar]
- Ross, M.T.; LaBrie, S.; McPherson, J.; Stanton, V.P. Screening large-insert libraries by hybridization. Curr. Prot. Hum. Genet. 2001. [Google Scholar] [CrossRef]
- Schummer, M.; Ng, V.L.V.; Baumgarner, R.E.; Nelson, P.S.; Schummer, B.; Bednarski, D.W.; Hassell, L.; Baldwin, R.L.; Karlan, B.Y.; Hood, L. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene 1999, 238, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, R.; Menossi, M. Pipeline for macro- and microarray analyses. Braz. J. Med. Biol. Res. 2007, 40, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Drummond, R.; Pinheiro, A.; Rocha, C.; Menossi, M. ISER: Selection of differentially expressed genes from DNA array data by non-linear data transformations and local fitting. Bioinformatics 2005, 21, 4427–4429. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]

| Sugarcane Clone Identification a | E-Value | Identity (%) | BLAST Hit b | Description c |
|---|---|---|---|---|
| SacBBI1 GI: 34966865 GB: CA113558.1 | 2 × 10−24 | 73/87 (84%) | GI:195610004 | Bowman–Birk type wound-induced proteinase inhibitor WIP1 precursor [Zea mays] |
| SacBBI2 GI: 35951517 GB: CA261007.1 | 1 × 10−40 | 85/98 (87%) | GI:115434342 | Bowman–Birk type proteinase inhibitor Oryza sativa Japonica Group |
| SacBBI3 GI: 35965021 GB: CA266304.1 | 5 × 10−43 | 74/88 (84%) | GI:195610814 | Bowman–Birk type bran trypsin inhibitor precursor [Zea mays] |
| SacBBI4 GI: 35984624 GB: CA272687.1 | 2 × 10−39 | 85/98 (87%) | GI:115434342 | Bowman–Birk type proteinase inhibitor Oryza sativa Japonica Group |
| SacMPI-like1 GI: 34922345 GB: CA070500.1 | 4 × 10−26 | 51/60 (85%) | GI:214015177 | Maize proteinase inhibitor [Zea mays subsp. parviglumis] |
| SacMPI-like2 GI: 35258606 GB: CA212876.1 | 6 × 10−33 | 59/66 (89%) | GI:75994161 | Maize protease inhibitor [Zea mays subsp. parviglumis] |
| SacMPIlike3 GI: 36014330 GB:CA282462.1 | 1 × 10−31 | 58/70 (83%) | GI:214015219 | Maize proteinase inhibitor [Zea mays subsp. parviglumis] |
| SacMPI-like4 GI: 36037506 GB: CA288211.1 | 9 × 10−29 | 55/65 (85%) | GI:214015093 | Maize proteinase inhibitor [Zea mays subsp. parviglumis] |
| SacMPI-like5 GI: 36065043 GB:CA297188.1 | 2 × 10−28 | 56/65 (85%) | GI:214015177 | Maize proteinase inhibitor [Zea mays subsp. parviglumis] |
| SacCI-1B-like GI: 35010896 GB: CA129230.1 | 0.067 | 17/26 (65%) | GI:226507138 | Subtilisin-chymotrypsin inhibitor CI-1B [Zea mays] |
| SacClp-like1 CA136349.1 | 2 × 10−77 | 94/94 (100%) | GI:242061800 | Clp amino terminal domain; Sorghum bicolor |
| SacChy-like GI: 35946811 GB: CA258670.1 | 1 × 10−153 | 215/221 (97%) | GI:242077536 | PDZ domain of trypsin-like serine proteases |
| SacCPD-like1 GI: 36002999 GB: CA278685.1 | No significant similarity found |
| Sugarcane Clone Identification a | E-Value | Identity (%) | BLAST Hit b | Description c |
|---|---|---|---|---|
| SacClp-like2 GI: 34940929 GB: CA087622.1 | 1 × 10−137 | 192/198 (97%) | GI:195612324 | ATP-dependent Clp protease proteolytic subunit 2 [Zea mays] |
| SacClp-like3 GI: 34966311 GB: CA113004.1 | 4 × 10−143 | 208/224 (93%) | GI:413935895 | Putative chaperone clp family protein [Zea mays] |
| SacClp-like4 GI: 35005555 GB: CA126553.1 | 5 × 10−62 | 131/150 (87%) | GI:347602486 | ATP-dependent Clp protease ATP-binding subunit ClpC homolog 1, Oryza sativa Japonica Group |
| SacClp-like5 GI: 35081269 GB: CA164148.1 | 7 × 10−108 | 180/189 (95%) | GI:475585607 | ATP-dependent Clp protease ATP-binding subunit clpA-like CD4A protein, chloroplastic [Aegilops tauschii] |
| SacCPD-like2 GI: 34966324 GB: CA113017.1 | 4 × 10−127 | 177/198 (89%) | GI:195637388 | Serine carboxypeptidase K10B2.2 precursor [Zea mays] |
| SacCPD-like3 GI: 35050806 GB: CA149102.1 | 7 × 10−58 | 94/105 (90%) | GI:226507958 | Serine carboxypeptidase K10B2.2 precursor [Zea mays] |
| SacSub-like1 GI: 34948297 GB: CA094990.1 | 2 × 10−136 | 199/221 (90%) | GI:414880317 | TPA: putative subtilase family protein [Zea mays] |
| SacSub-like2 GI: 34967468 GB: CA114161.1 | 2 × 10−42 | 80/100 (80%) | GI:42407651 | Putative subtilisin-like proteinase [Oryza sativa Japonica Group] |
| SacSub-like3 GI: 34967945 GB: CA114638.1 | 6 × 10−76 | 124/158 (78%) | GI:475577050 | Subtilisin-like protease [Aegilops tauschii] |
| Role | Arabidopsis Subunit | Accession | Sugarcane EST Homologue | Expression Profile * |
|---|---|---|---|---|
| Clp AAA+ chaperones | ClpC1 | AT5G50920 | CA119085.1 | - |
| CA124181.1 | - | |||
| CA126553.1 (SacClp-like4) | −1.9 ± 0.07 | |||
| CA132637.1 | - | |||
| CA164148.1 (SacClp-like5) | −1.9 ± 0.06 | |||
| ClpC2 | AT3G48870 | CA113004.1 (SacClp-like3) | −6.4 ± 0.03 | |
| ClpD | AT5G51070 | CA119462.1 | - | |
| CA136349.1 (SacClp-like1) | 27,11 ± 10,31 | |||
| CA145821.1 | - | |||
| CA194919.1 | - | |||
| CA212375.1 | - | |||
| serine-type ClpP | ClpP1 | ATCG00670 | CA119497.1 | - |
| ClpP3 | AT1G66670 | CA119729.1 | - | |
| ClpP4 | AT5G45390 | CA107353.1 | - | |
| ClpP5 | AT1G02560 | CA074329.1 | - | |
| CA183086.1 | - | |||
| ClpP6 | AT1G11750 | CA282400.1 | - | |
| ClpP7 | AT5G23140 | - | - | |
| ClpP-related ClpR | ClpR1 | AT1G49970 | CA108609.1 | - |
| CA108695.1 | - | |||
| ClpR2 | AT1G12410 | CA087622.1 (SacClp-like2) | −2.1 ± 0.14 | |
| ClpR3 | AT1G09130 | Not found | ||
| ClpR4 | AT4G17040 | CA113615.1 | - |
| Sugarcane Clone Name | GenBank Accession Number | Relative mRNA Level | p Value |
|---|---|---|---|
| SacClp-like2 | CA087622.1 | ‒2.1 ± 0.14 | p < 0.005 |
| SacClp-like3 | CA113004.1 | ‒6.4 ± 0.03 | p < 0.005 |
| SacClp-like4 | CA126553.1 | ‒1.9 ± 0.07 | p < 0.005 |
| SacClp-like5 | CA164148.1 | ‒1.9 ± 0.06 | p < 0.005 |
| SacCPD-like2 | CA113017.1 | ‒2.1 ± 0.07 | p < 0.005 |
| SacCPD-like3 | CA149102.1 | ‒44.2 ± 0.008 | p < 0.005 |
| SacSub-like1 | CA094990.1 | ‒4.4 ± 0.04 | p < 0.005 |
| SacSub-like2 | CA114161.1 | ‒2.7 ± 0.08 | p < 0.005 |
| SacSub-like3 | CA114638.1 | ‒1.6 ± 0.06 | p < 0.005 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, A.H.; Mingossi, F.B.; Dias, R.O.; Franco, F.P.; Vicentini, R.; Mello, M.O.; Moura, D.S.; Silva-Filho, M.C. Sugarcane Serine Peptidase Inhibitors, Serine Peptidases, and Clp Protease System Subunits Associated with Sugarcane Borer (Diatraea saccharalis) Herbivory and Wounding. Int. J. Mol. Sci. 2016, 17, 1444. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091444
Medeiros AH, Mingossi FB, Dias RO, Franco FP, Vicentini R, Mello MO, Moura DS, Silva-Filho MC. Sugarcane Serine Peptidase Inhibitors, Serine Peptidases, and Clp Protease System Subunits Associated with Sugarcane Borer (Diatraea saccharalis) Herbivory and Wounding. International Journal of Molecular Sciences. 2016; 17(9):1444. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091444
Chicago/Turabian StyleMedeiros, Ane H., Fabiana B. Mingossi, Renata O. Dias, Flávia P. Franco, Renato Vicentini, Marcia O. Mello, Daniel S. Moura, and Marcio C. Silva-Filho. 2016. "Sugarcane Serine Peptidase Inhibitors, Serine Peptidases, and Clp Protease System Subunits Associated with Sugarcane Borer (Diatraea saccharalis) Herbivory and Wounding" International Journal of Molecular Sciences 17, no. 9: 1444. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091444