New Phase of Growth for Xenogeneic-Based Bioartificial Organs
Abstract
:1. Introduction
2. Bioartificial Organs on the Market
3. Representative Examples of Bioartificial Organs
3.1. Organ Bio-Printing
3.2. Scaffold Re-Cellularization
3.2.1. Bioartificial Bladder
3.2.2. Bioartificial Trachea
3.3. Optimization of Cellular Repair/Regeneration
3.3.1. Bioartificial Kidney
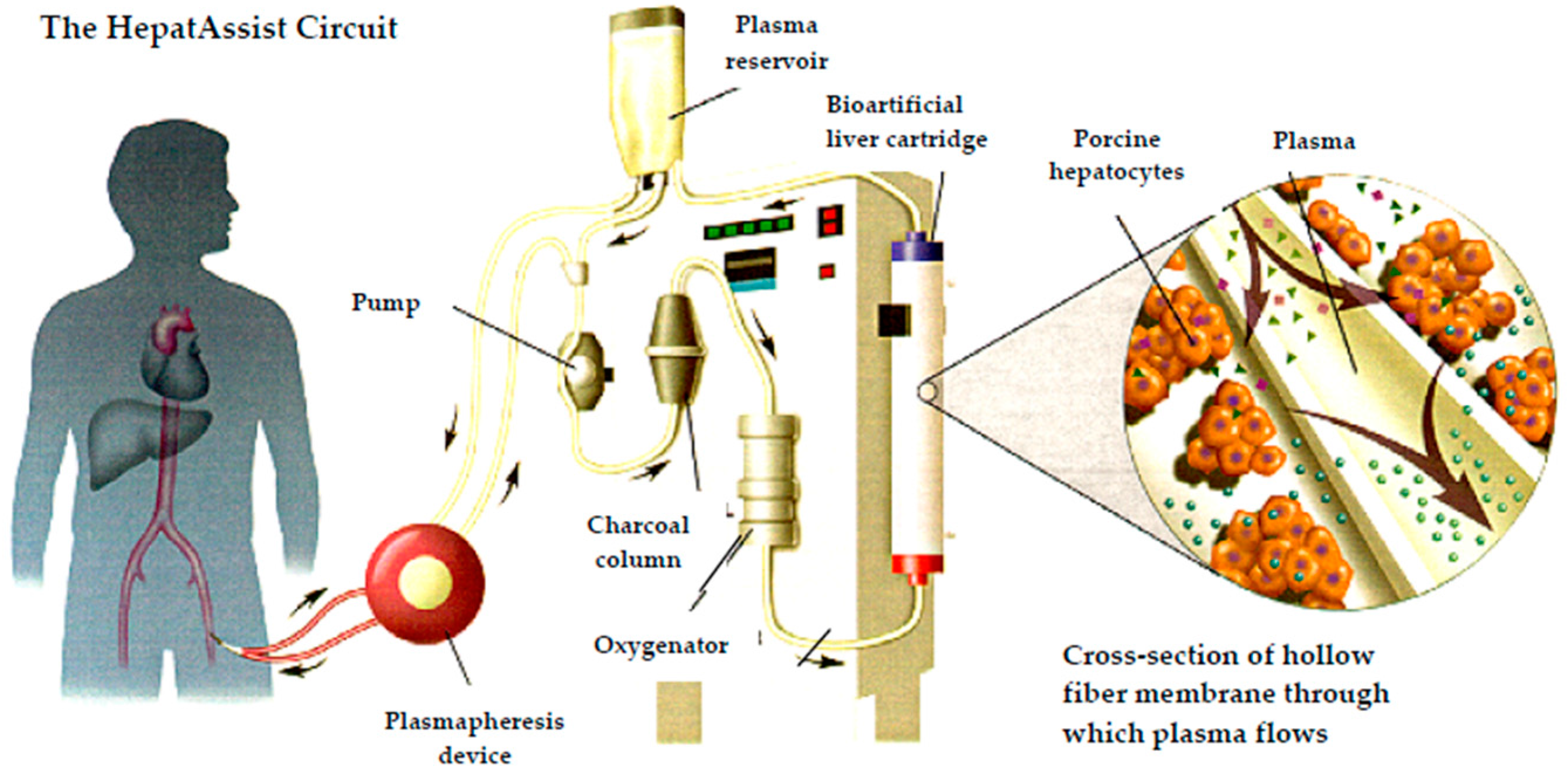
3.3.2. Bioartificial Liver
4. Bioartificial Organs and Xenotransplantation
4.1. Risks in Xenotransplantation
4.1.1. Immunological Barriers
4.1.2. Risk of Xenozoonosis
4.1.3. PERV Infection: Risk Assessment
Severity
Detectability
Likelihood
Overall Risk Assessment
5. Conclusions
Conflicts of Interest
References
- UNOS. Available online: https://www.unos.org/data (accessed on 20 May 2016).
- Orlando, G.; Soker, S.; Stratta, R.J. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Ann. Surg. 2013, 258, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Ash, S.R. Artificial organs: A new chapter in medical history. ASAIO J. 2006, 52, e3–e9. [Google Scholar] [CrossRef] [PubMed]
- Malchesky, P.S. Artificial organs and vanishing boundaries. Artif. Organ. 2001, 25, 75–88. [Google Scholar] [CrossRef]
- Malchesky, P.S. Artificial Organs 2015: A year in review. Artif. Organ. 2016, 40, 294–321. [Google Scholar] [CrossRef] [PubMed]
- Pless, G. Artificial and bioartificial liver support. Organogenesis 2007, 3, 20–24. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 8 June 2016).
- Orlando, G.; Baptista, P.; Birchall, M.; de Coppi, P.; Farney, A.; Guimaraes-Souza, N.K.; Opara, E.; Rogers, J.; Seliktar, D.; Shapira-Schweitzer, K.; et al. Regenerative medicine as applied to solid organ transplantation: Current Status and future challenges. Transplant Int. 2011, 24, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Peloso, A.; Dhal, A.; Zambon, J.P.; Li, P.; Orlando, G.; Atala, A.; Soker, S. Current achievements and future perspectives in whole-organ bioengineering. Stem Cell Res. Ther. 2015, 6, 1–12. [Google Scholar]
- Li, H.; Chen, H.S.; Nyberg, S.L. Extracorporeal liver support and liver transplant for patients with acute-on-chronic liver failure. Semin. Liver Dis. 2016, 36, 153–160. [Google Scholar] [PubMed]
- Humes, H.D.; Buffington, D.; Westover, A.J.; Roy, S.; Fissell, W.H. The bioartificial kidney: Current status and future promise. Pediat. Nephrol. 2014, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Veves, A.; Falanga, V.; Armstrong, D.G.; Sabolinski, M.L. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers a prospective randomized multicenter clinical trial. Diabetes Care 2001, 24, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Marston, W.A.; Hanft, J.; Norwood, P.; Pollak, R. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers results of a prospective randomized trial. Diabetes Care 2003, 26, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Sabolinski, M. A bilayered living skin construct (APLIGRAF®) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999, 7, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Stone, R.; Rosa, M.; Ramirez, H.; Badiavas, E.; Blumenberg, M.; Tomic-Canic, M. Treatment with Bilayered Living Cellular Construct Triggers Acute Healing Response in Non-healing Venous Leg Ulcers (Poster LB-046). In Proceedings of the Organogenesis’ Expanding Wound Care Portfolio Featured in Eight New Presentations this Week at SAWC/WHS Spring 2016, Atlanta, GA, USA, 14 April 2016.
- Hunsberger, J.; Neubert, J.; Wertheim, J.A.; Allickson, J.; Atala, A. Bioengineering priorities on a path to ending organ shortage. Curr. Stem Cell Rep. 2016, 2, 118–127. [Google Scholar] [CrossRef]
- Rezende, R.A.; Kasyanov, V.; Mironov, V.; da Silva, J.V.L. Organ printing as an information technology. Procedia Eng. 2015, 110, 151–158. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, M.; Peloso, A.; Katari, R.; Soker, S.; Lerut, J.P.; Stratta, R.J.; Orlando, G. Semi-xenotransplantation: The regenerative medicine-based approach to immunosuppression-free transplantation and to meet the organ demand. Xenotransplantation 2015, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, C.; Latancia, M.T.; Otterbein, L.E.; Netti, P.A. Update on renal replacement therapy: Implantable artificial devices and bioengineered organs. Tissue Eng. Part B Rev. 2016, 24, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Atala, A. Bioengineered tissues for urogenital repair in children. Pediatr. Res. 2008, 63, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P.; et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008, 372, 2023–2030. [Google Scholar] [CrossRef]
- Jungebluth, P.; Alici, E.; Baiguera, S.; Le Blanc, K.; Blomberg, P.; Bozóky, B.; Crowley, C.; Einarsson, O.; Grinnemo, K.H.; Gudbjartsson, T.; et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: A proof-of-concept study. Lancet 2011, 378, 1997–2004. [Google Scholar] [CrossRef]
- Gonfiotti, A.; Jaus, M.O.; Barale, D.; Baiguera, S.; Comin, C.; Lavorini, F.; Fontana, G.; Sibila, O.; Rombolà, G.; Jungebluth, P.; et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet 2014, 383, 238–244. [Google Scholar] [CrossRef]
- Cyranoski, D. Karolinska Institute to cut ties with controversial surgeon. Nature 2016. [Google Scholar] [CrossRef]
- Humes, H.D.; MacKay, S.M.; Funke, A.J.; Buffington, D.A. The bioartificial renal tubule assist device to enhance CRRT in acute renal failure. Am. J. Kidney Dis. 1997, 30, S28–S31. [Google Scholar] [CrossRef]
- Humes, H.D.; Fissell, W.H.; Weitzel, W.F.; Buffington, D.A.; Westover, A.J.; MacKay, S.M.; Gutierrez, J.M. Metabolic replacement of kidney function in uremic animals with a bioartificial kidney containing human cells. Am. J. Okidney Dis. 2002, 39, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Buffington, D.A.; Westover, A.J.; Johnston, K.A.; Humes, H.D. The bioartificial kidney. Transl. Res. 2014, 163, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Humes, H.D.; Weitzel, W.F.; Bartlett, R.H.; Swaniker, F.C.; Paganini, E.P.; Luderer, J.R.; Sobota, J. Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int. 2004, 66, 1578–1588. [Google Scholar] [PubMed]
- Tumlin, J.; Wali, R.; Williams, W.; Murray, P.; Tolwani, A.J.; Vinnikova, A.K.; Szerlip, H.M.; Ye, J.; Paganini, E.P.; Dworkin, L.; et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J. Am. Soc. Nephrol. 2008, 19, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.L. Bridging the gap: Advances in artificial liver support. Liver Transplant. 2012, 18, S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Pless, G. Bioartificial liver support systems. In Hepatocytes; Springer: New York, NY, USA, 2010; pp. 511–523. [Google Scholar]
- Wood, R.; Katz, S.M.; Ozaki, C.F.; Monsour, H.P.; Gislason, G.T.; Kelly, J.H.; Sussman, N.L. Extracorporeal liver assist device (ELAD). In Transplantation Proceedings; Elsevier: Houston, TX, USA, 1993; pp. 53–54. [Google Scholar]
- Gislason, G.T.; Lobdell, D.D.; Kelly, J.H.; Sussman, N.L. A treatment system for implementing an extracorporeal liver assist device. Artif. Organ. 1994, 18, 385–389. [Google Scholar]
- Ellis, A.J.; Hughes, R.D.; Wendon, J.A.; Dunne, J.; Langley, P.G.; Kelly, J.H.; Gislason, G.T.; Sussman, N.L.; Williams, R. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Int. Hepatol. Commun. 1997, 7, 140. [Google Scholar] [CrossRef]
- Demetriou, A.A.; Rozga, J.; Podesta, L.; Lepage, E.; Morsiani, E.; Moscioni, A.D.; Hoffman, A.; McGrath, M.; Kong, L.; Rosen, H.; et al. Early clinical experience with a hybrid bioartificial liver. Scand. J. Gastroenterol. Suppl. 1995, 208, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mullon, C.; Pitkin, Z. The HepatAssist® Bioartificial Liver Support System: Clinical study and pig hepatocyte process. Expert Opin. Investig. Drugs 1999, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.D.; Mullon, C.J.; Hewitt, W.R.; Arkadopoulos, N.; Kahaku, E.; Eguchi, S.; Khalili, T.; Arnaout, W.; Shackleton, C.R.; Rozga, J.; et al. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann. Surg. 1997, 225, 484. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, A.A.; Brown, R.S., Jr.; Busuttil, R.W.; Fair, J.; McGuire, B.M.; Rosenthal, P.; Am Esch, J.S., II; Lerut, J.; Nyberg, S.L.; Salizzoni, M.; et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann. Surg. 2004, 239, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, Z.; Mullon, C. Evidence of absence of porcine endogenous retrovirus (PERV) infection in patients treated with a bioartificial liver support system. Artif. Organ. 1999, 23, 829–833. [Google Scholar]
- Pitkin, Z.; Switzer, W.; Chapman, L. An interim analysis of PERV infectivity in 74 patients treated with a bioartificial liver in a prospective, randomized, multicenter controlled trial. Hepatology 2001, 34, 249A. [Google Scholar]
- Gerlach, J. Development of a hybrid liver support system: A review. Int. J. Artif. Organ. 1996, 19, 645–654. [Google Scholar]
- Sauer, I.M.; Kardassis, D.; Zeillinger, K.; Pascher, A.; Gruenwald, A.; Pless, G.; Irgang, M.; Kraemer, M.; Puhl, G.; Frank, J.; et al. Clinical extracorporeal hybrid liver support–phase I study with primary porcine liver cells. Xenotransplantation 2003, 10, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Irgang, M.; Sauer, I.M.; Karlas, A.; Zeilinger, K.; Gerlach, J.C.; Kurth, R.; Neuhaus, P.; Denner, J. Porcine endogenous retroviruses: No infection in patients treated with a bioreactor based on porcine liver cells. J. Clin. Virol. 2003, 28, 141–154. [Google Scholar] [CrossRef]
- Glorioso, J.M.; Mao, S.A.; Rodysill, B.; Mounajjed, T.; Kremers, W.K.; Elgilani, F.; Hickey, R.D.; Haugaa, H.; Rose, C.F.; Amiot, B.; et al. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J. Hepatol. 2015, 63, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry: Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans (2003). Available online: http://www.fda.gov/BiologicsBloodVaccines/guidancecomplianceregulatoryinformation/Guidances/Xenotransplantation/ucm074354.htm (accessed on 30 August 2016).
- Cooper, D.K.; Ekser, B.; Tector, A.J. A brief history of clinical xenotransplantation. Int. J. Surg. 2015, 23, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Fung, J.; Tzakis, A.; Todo, S.; Demetris, A.J.; Marino, I.R.; Doyle, H.; Zeevi, A.; Warty, V.; Kusne, S.; et al. Baboon-to-human liver transplantation. Lancet 1993, 341, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Qi, M. Transplantation of encapsulated pancreatic islets as a treatment for patients with type 1 diabetes mellitus. Adv. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. Xenotransplantation-progress and problems: A review. J. Transplant. Technol. Res. 2014. [Google Scholar] [CrossRef]
- Samdani, T.; Greenstein, S.; Atray, N.; Vachharajani, T.; Chao, E.; Talavera, F.; Sudan, D.; Shapiro, R. Xenotransplantation. Available online: http://emedicine.medscape.com/article/432418-overview (accessed on 30 August 2016).
- Byrne, G.W.; McCurry, K.R.; Martin, M.J.; McClellan, S.M.; Platt, J.L.; Logan, J.S. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage1. Transplantation 1997, 63, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tector, J. New hope for liver xenotransplantation. Ann. Surg. 2016, 263, 1072. [Google Scholar] [CrossRef] [PubMed]
- Perkel, J.M. Xenotransplantation makes a comeback. Nat. Biotechnol. 2016, 34, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Estrada, J.L.; Burlak, C.; Montgomery, J.; Butler, J.R.; Santos, R.M.; Wang, Z.-Y.; Paris, L.L.; Blankenship, R.L.; Downey, S.M.; et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation 2015, 22, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ekser, B.; Cooper, D.K.; Tector, A.J. The need for xenotransplantation as a source of organs and cells for clinical transplantation. Int. J. Surg. 2015, 23, 199–204. [Google Scholar]
- Perota, A.; Lagutina, I.; Quadalti, C.; Lazzari, G.; Cozzi, E.; Galli, C. The applications of genome editing in xenotransplantation. J. Genet. Genom. 2016, 43, 233–237. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Ekser, B.; Ramsoondar, J.; Phelps, C.; Ayares, D. The role of genetically engineered pigs in xenotransplantation research. J. Pathol. 2016, 238, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Dai, Y.; Mou, L.; Cooper, D.K.; Shi, D.; Cai, Z. The potential of the combination of CRISPR/Cas9 and pluripotent stem cells to provide human organs from chimaeric pigs. Int. J. Mol. Sci. 2015, 16, 6545–6556. [Google Scholar] [CrossRef] [PubMed]
- Duran-Struuck, R.; Lutton, B.V. Xenotransplantation and transgenic technologies. In Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques; CRC Press: Boca Raton, FL, USA, 2015; pp. 405–418. [Google Scholar]
- Nottle, M.B.; Hawthorne, W.; O’Connell, P.J.; d’Apice, A.J.F.; Cowan, P.J. Xenotransplantation Transgenesis: Are we there yet? Cloning Transgenes. 2015. [Google Scholar] [CrossRef]
- Garkavenko, O.; Muzina, M.; Muzina, Z.; Powels, K.; Elliott, R.B.; Croxson, M.C. Monitoring for potentially xenozoonotic viruses in New Zealand pigs. J. Med. Virol. 2004, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Patience, C.; Takeuchi, Y.; Weiss, R.A. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 1997, 3, 282–286. [Google Scholar]
- Fishman, J.A.; Scobie, L.; Takeuchi, Y. Xenotransplantation-associated infectious risk: A WHO consultation. Xenotransplantation 2012, 19, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C. Endogenous retroviruses. Cell. Mol. Life Sci. 2008, 65, 3399–3412. [Google Scholar] [CrossRef] [PubMed]
- WHO. OECD/WHO Consultation on Xenotransplantation Surveillance: Summary. 2001. Available online: http://www.who.int/transplantation/publications/OECD_WHO.pdf (accessed on 31 August 2016).
- WHO. Second WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials. 17–19 October 2011, Geneva, Switzerland. Available online: http://www.who.int/transplantation/xeno/report2nd_global_consultation_xtx.pdf (accessed on 30 August 2016).
- Denner, J.; Tönjes, R.R.; Takeuchi, Y.; Fishman, J.; Scobie, L. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 5: Recipient monitoring and response plan for preventing disease transmission. Xenotransplantation 2016, 23, 53–59. [Google Scholar] [PubMed]
- Reardon, S. New life for pig organs. Nature 2015, 527, 152–154. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Guidance for Industry: Q9 Quality Risk Management (2006). Available online: http://www.gmp-compliance.org/eca_guideline_388.html (accessed on 30 August 2016).
- Argaw, T.; Colon-Moran, W.; Wilson, C. Susceptibility of porcine endogenous retrovirus to anti-retroviral inhibitors. Xenotransplantation 2016, 23, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Güell, M.; Niu, D.; George, H.; Lesha, E.; Grishin, D.; Aach, J.; Shrock, E.; Xu, W.; Poci, J.; et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 2015, 350, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Salomon, D.R. A CRISPR way to block PERVs—Engineering organs for transplantation. N. Engl. J. Med. 2016, 374, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.B.; Escobar, L.; Garkavenko, O.; Croxson, M.C.; Schroeder, B.A.; McGregor, M.; Ferguson, G.; Beckman, N.; Ferguson, S. No evidence of infection with porcine endogenous retrovirus in recipients of encapsulated porcine islet xenografts. Cell Transplant. 1999, 9, 895–901. [Google Scholar]
- Paradis, K.; Langford, G.; Long, Z.; Heneine, W.; Sandstrom, P.; Switzer, W.M.; Chapman, L.E.; Lockey, C.; Onions, D.; Otto, E.; et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science 1999, 285, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Patience, C.; Patton, G.S.; Takeuchi, Y.; Weiss, R.A.; McClure, M.O.; Rydberg, L.; Breimer, M.E. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet 1998, 352, 699–701. [Google Scholar] [CrossRef]
- Garkavenko, O.; Croxson, M.C.; Irgang, M.; Karlas, A.; Denner, J.; Elliott, R.B. Monitoring for presence of potentially xenotic viruses in recipients of pig islet xenotransplantation. J. Clin. Microbiol. 2004, 42, 5353–5356. [Google Scholar]
- Di Nicuolo, G.; D’Alessandro, A.; Andria, B.; Scuderi, V.; Scognamiglio, M.; Tammaro, A.; Mancini, A.; Cozzolino, S.; Di Florio, E.; Bracco, A.; et al. Long-term absence of porcine endogenous retrovirus infection in chronically immunosuppressed patients after treatment with the porcine cell–based Academic Medical Center bioartificial liver. Xenotransplantation 2010, 17, 431–439. [Google Scholar] [CrossRef] [PubMed]
- PDA Technical Report 54-3. Implementation of Quality Risk Management for Pharmaceutical and Biotechnology Manufacturing Operations. Available online: https://store.pda.org/tableofcontents/tr54-213_toc.pdf (accessed on 30 August 2016).


| Targeted Disease Indication | BAO 1 System Name | Category of Xenotransplantation Product | Source of Cells/Tissues | Type of Exposure | Number of Patients | PERV Detected Yes/No | Reference |
|---|---|---|---|---|---|---|---|
| Liver failure | BLSS | Extracorporeal liver support system | Primary pig liver cells | Membrane bioreactor | 5 | No | [65] |
| AMC-BAL | Extracorporeal liver support system | Primary pig liver cells | Membrane bioreactor | 12 | No | [41] | |
| RFB | Extracorporeal liver support system | Primary pig liver cells | Membrane bioreactor | 7 | No | [40] | |
| MELS | Extracorporeal hybrid liver support system | Primary pig liver cells | Membrane bioreactor | 8 | No | [36,37] | |
| HepatAssist | Extracorporeal liver support system | Cryopreserved pig liver cells | Membrane bioreactor | 103 | No | [33,34] | |
| – | Extracorporeal pig liver perfusion | Transgenic pig liver | Direct exposure | 2 | No | [66] | |
| Chronic Glomerulonephritis | – | Extracorporeal pig kidney perfusion | Pig kidney | Direct exposure | 2 | No | [67] |
| Neurological conditions 2 | – | Direct transplantation | Cells from fetal pigs | Direct exposure | 24 | No | [68] |
| Diabetes | DIABECEll® | Alginate-encapsulated cells | Porcine Islet cell Tx 3 | 16 | No | [69,70] | |
| – | Porcine islet cell Tx 3 | Direct exposure | 10 | No | [71] | ||
| Various indications | – | Extracorporeal pig organ perfusion, pig islets | Pig kidney, liver, spleen, islets 4 | Direct exposure | 160 | No | [72] |
| Severity | Likelihood | ||
| Low | Medium | High | |
| High | Class 2 | Class 1 | Class 1 |
| Medium | Class 3 | Class 2 | Class 1 |
| Low | Class 3 | Class 3 | Class 2 |
| Risk Class | Detectability | ||
| Low | Medium | High | |
| Class 1 | High risk | High risk | Medium risk |
| Class 2 | High risk | Medium risk | Low risk |
| Class 3 | Medium risk | Low risk | Low risk |
| Severity | Likelihood | ||
| Low | Medium | High | |
| High | Class 2 | Class 1 | Class 1 |
| Medium | Class 3 | Class 2 | Class 1 |
| Low | Class 3 | Class 3 | Class 2 |
| Risk Class | Detectability | ||
| Low | Medium | High | |
| Class 1 | High risk | High risk | Medium risk |
| Class 2 | High risk | Medium risk | Low risk |
| Class 3 | Medium risk | Low risk | Low risk |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitkin, Z. New Phase of Growth for Xenogeneic-Based Bioartificial Organs. Int. J. Mol. Sci. 2016, 17, 1593. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091593
Pitkin Z. New Phase of Growth for Xenogeneic-Based Bioartificial Organs. International Journal of Molecular Sciences. 2016; 17(9):1593. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091593
Chicago/Turabian StylePitkin, Zorina. 2016. "New Phase of Growth for Xenogeneic-Based Bioartificial Organs" International Journal of Molecular Sciences 17, no. 9: 1593. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091593






