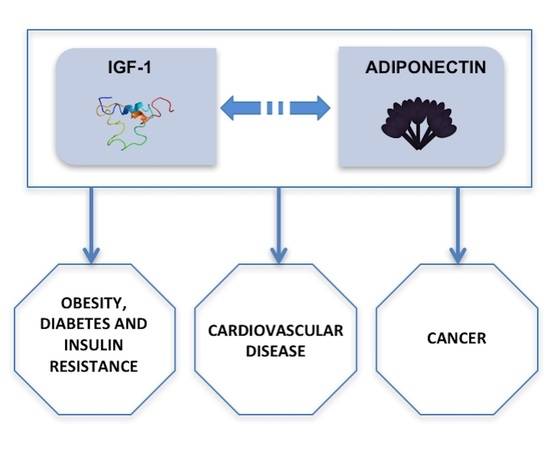
A Functional Interplay between IGF-1 and Adiponectin
Abstract
:1. Introduction
2. Insulin-like growth factor-1 (IGF-1)
3. Adiponectin
4. IGF-1 and Adiponectin in Relation to Obesity, Diabetes, and Insulin Resistance
5. IGF-1 and Adiponectin in Relation to Cardiovascular Diseases
6. IGF-1 and Adiponectin in Relation to Cancer
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Puche, J.E.; Castilla-Cortázar, I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J. Transl. Med. 2012, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, M.; Okada-Iwabu, M.; Yamauchi, T.; Kadowaki, T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech. Dis. 2015, 1, 15013–15018. [Google Scholar] [CrossRef] [PubMed]
- Imperlini, E.; Mancini, A.; Alfieri, A.; Martone, D.; Caterino, M.; Orrù, S.; Buono, P. Molecular effects of supraphysiological doses of doping agents on health. Mol. BioSyst. 2015, 11, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. The insulin and insulin-like growth factor receptor familyin neoplasia: An update. Nat. Rev. Cancer 2012, 12, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A.; Kwasniewski, W.; Adamek, A.; Gozdzicka-Jozefiak, A. Insulin-like growth factor (IGF) axis in cancerogenesis. Mutat. Res. 2017, 772, 78–104. [Google Scholar] [CrossRef] [PubMed]
- Laviola, L.; Natalicchio, A.; Giorgino, F. The IGF-I signaling pathway. Curr. Pharm. Des. 2007, 13, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Firth, S.M.; Baxter, R.C. Cellular Actions of the Insulin-Like Growth Factor Binding Proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.D.; Griffiths, A.M. Mechanisms of growth impairment in pediatric Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 2009, 21, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Visser, J.; Huang, G.S. IGF2 signaling and regulation in cancer. Cytokine Growth Factor Rev. 2015, 26, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, C.E., Jr.; Custodio, R.J.; Aguiar-Oliveira, M.E. Physiology of the GH-IGF axis. Arq. Bras. Endocrinol. Metabol. 2008, 52, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Iams, W.T.; Lovly, C.M. Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade. Clin. Cancer Res. 2015, 21, 4270–4277. [Google Scholar] [CrossRef] [PubMed]
- McKoy, G.; Ashley, W.; Mander, J.; Yang, S.Y.; Williams, N.; Russell, B.; Goldspink, G. Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J. Physiol. 1999, 516, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, F.; Yeung, E.W.; Li, Y. IGF-IEc expression, regulation and biological function in different tissues. Growth Horm. IGF Res. 2010, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A.; Headey, S.J.; Norton, R.S. IGF-binding proteins—The pieces are falling into place. Trends Endocrinol. Metab. 2005, 16, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat. Rev. Cancer 2014, 14, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Mazziotti, G.; Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008, 29, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Perrini, S.; Carreira, M.C.; Conserva, A.; Laviola, L.; Giorgino, F. Metabolic implications of growth hormone therapy. J. Endocrinol. Investig. 2008, 31, 79–84. [Google Scholar]
- Perrini, S.; Natalicchio, A.; Laviola, L.; Cignarelli, A.; Melchiorre, M.; De Stefano, F.; Caccioppoli, C.; Leonardini, A.; Martemucci, S.; Belsanti, G.; et al. Abnormalities of insulin-like growth factor-I signaling and impaired cell proliferation in osteoblasts from subjects with osteoporosis. Endocrinology 2008, 149, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Møller, N.; Jørgensen, J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Moller, N.; Bigelow, M.L.; Coenen-Schimke, J.; Nair, K.S. Enhancement of muscle mitochondrial function by growth hormone. J. Clin. Endocrinol. Metab. 2008, 93, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Guha, N.; Dashwood, A.; Thomas, N.J.; Skingle, A.J.; Sönksen, P.H.; Holt, R.I. IGF-I abuse in sport. Curr. Drug Abus. Rev. 2009, 2, 263–272. [Google Scholar] [CrossRef]
- Baumann, G.P. Growth hormone doping in sports: A critical review of use and detection strategies. Endocr. Rev. 2012, 33, 155–186. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.K.; Gravholt, C.H.; ØRskov, H.; Rasmussen, M.H.; Christiansen, J.S.; Jørgensen, J.O. Dose dependency of the pharmacokinetics and acute lipolytic actions of growth hormone. J. Clin. Endocrinol. Metab. 2002, 87, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Chikani, V.; Ho, K.K. Action of GH on skeletal muscle function: Molecular and metabolic mechanisms. J. Mol. Endocrinol. 2013, 52, R107–R123. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Imperlini, E.; Alfieri, A.; Spaziani, S.; Martone, D.; Parisi, A.; Orru, S.; Buono, P. DHT and IGF-1 in peripheral blood lymphocytes: New markers for the biological passport of athletes. J. Biol. Regul. Homeost. Agents 2013, 27, 757–770. [Google Scholar] [PubMed]
- Spaziani, S.; Imperlini, E.; Mancini, A.; Caterino, M.; Buono, P.; Orrù, S. Insulin-like growth factor 1 receptor signaling induced by supraphysiological doses of IGF-1 in human peripheral blood lymphocytes. Proteomics 2014, 14, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Imperlini, E.; Spaziani, S.; Mancini, A.; Caterino, M.; Buono, P.; Orrù, S. Synergistic effect of DHT and IGF-1 hyperstimulation in human peripheral blood lymphocytes. Proteomics 2015, 15, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Richmond, E.J.; Rogol, A.D. Growth hormone deficiency in children. Pituitary 2008, 11, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.; Auriemma, R.S.; Pivonello, R.; Colao, A. Cushing, acromegaly, GH deficiency and tendons. Muscles Ligaments Tendons J. 2014, 4, 29–32. [Google Scholar] [CrossRef]
- Mauras, N.; Martinez, V.; Rini, A.; Guevara-Aguirre, J. Recombinant human insulin-like growth factor I has significant anabolic effects in adults with growth hormone receptor deficiency: Studies on protein, glucose, and lipid metabolism. J. Clin. Endocrinol. Metab. 2000, 85, 3036–3042. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, C.; Scarano, E.; Savastano, S.; Savanelli, M.C.; Pivonello, R.; Colao, A. Cardiovascular alterations in adult GH deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Ferone, D.; Marzullo, P.; Lombardi, G. Systemic complications of acromegaly: Epidemiology, pathogenesis, and management. Endocr. Rev. 2004, 25, 102–152. [Google Scholar] [CrossRef] [PubMed]
- Palmeiro, C.R.; Anand, R.; Dardi, I.K.; Balasubramaniyam, N.; Schwarcz, M.D.; Weiss, I.A. Growth hormone and the cardiovascular system. Cardiol. Rev. 2012, 20, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Hallengren, E.; Almgren, P.; Engström, G.; Hedblad, B.; Persson, M.; Suhr, J.; Bergmann, A.; Melander, O. Fasting levels of high-sensitivity growth hormone predict cardiovascular morbidity and mortality: The Malmö Diet and Cancer study. J. Am. Coll. Cardiol. 2014, 64, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Kita, S.; Obata, Y.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; Nishizawa, H.; Funahashi, T.; Takagi, J.; et al. The unique prodomain of T-cadherin plays a key role in adiponectin binding with the essential extracellular cadherin repeats 1 and 2. J. Biol. Chem. 2017, 292, 7840–7849. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Fujishima, Y.; Maeda, N.; Mori, T.; Hirata, A.; Sekimoto, R.; Tsushima, Y.; Masuda, S.; Yamaoka, M.; Inoue, K.; et al. Positive feedback regulation between adiponectin and T-cadherin impacts adiponectin levels in tissue and plasma of male mice. Endocrinology 2015, 15, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Rajitha, B.; Aliya, S.; Kotipatruni, R.P.; Madanraj, A.S.; Hammond, A.; Park, D.; Chigurupati, S.; Alam, A.; Pattnaik, S. The role of adiponectin in obesity-associated female-specific carcinogenesis. Cytokine Growth Factor Rev. 2016, 31, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ziemke, F.; Mantzoros, C.S. Adiponectin in insulin resistance: Lessons from translational research. Am. J. Clin. Nutr. 2010, 91, 258S–261S. [Google Scholar] [CrossRef] [PubMed]
- Hada, Y.; Yamauchi, T.; Waki, H.; Tsuchida, A.; Hara, K.; Yago, H.; Miyazaki, O.; Ebinuma, H.; Kadowaki, T. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem. Biophys. Res. Commun. 2007, 356, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Kihara, S.; Arita, Y.; Okamoto, Y.; Maeda, K.; Kuriyama, H.; Hotta, K.; Nishida, M.; Takahashi, M.; Muraguchi, M.; et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000, 102, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Funahashi, T.; Bodkin, N.L.; Ortmeyer, H.K.; Arita, Y.; Hansen, B.C.; Matsuzawa, Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 2001, 50, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.S.; Funahashi, T.; Hanson, R.L.; Matsuzawa, Y.; Tanaka, S.; Tataranni, P.A.; Knowler, W.C.; Krakoff, J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002, 360, 57–58. [Google Scholar] [CrossRef]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Kroke, A.; Möhlig, M.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003, 361, 226–268. [Google Scholar] [CrossRef]
- Bajaj, M.; Suraamornkul, S.; Piper, P.; Hardies, L.J.; Glass, L.; Cersosimo, E.; Pratipanawatr, T.; Miyazaki, Y.; DeFronzo, R.A. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2004, 89, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Pajvani, U.B.; Hawkins, M.; Combs, T.P.; Rajala, M.W.; Doebber, T.; Berger, J.P.; Wagner, J.A.; Wu, M.; Knopps, A.; Xiang, A.H.; et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 2004, 279, 12152–12162. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Horikoshi, M.; Yamauchi, T.; Yago, H.; Miyazaki, O.; Ebinuma, H.; Imai, Y.; Nagai, R.; Kadowaki, T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 2006, 29, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castro, C.; Luo, N.; Wallace, P.; Klein, R.L.; Garvey, W.T. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabete 2006, 55, 249–259. [Google Scholar] [CrossRef]
- Wei, T.; Ye, P.; Peng, X.; Wu, L.L.; Yu, G.Y. Circulating adiponectin levels in various malignancies: An updated meta-analysis of 107 studies. Oncotarget 2016, 7, 48671–48691. [Google Scholar] [CrossRef] [PubMed]
- Pardina, E.; Ferrer, R.; Baena-Fustegueras, J.A.; Lecube, A.; Fort, J.M.; Vargas, V.; Catalán, R.; Peinado-Onsurbe, J. The relationships between IGF-1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes. Surg. 2010, 20, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hu, Y.; Liu, C.; Qi, J.; Li, G. Low insulin-like growth factor 1 is associated with low high-density lipoprotein cholesterol and metabolic syndrome in Chinese nondiabetic obese children and adolescents: A cross-sectional study. Lipids Health Dis. 2016, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, A.; Gologan, S.; Arbanas, T.; Copaescu, C.; Martin, S.; Albu, A.; Barbu, C.; Pirvulescu, I.; Fica, S. Adiponectin, body mass index and hepatic steatosis are independently associated with IGF-1 status in obese non-diabetic women. Growth Horm. IGF Res. 2013, 23, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Selcuk, M.T.; Selcuk, H.; Temizhan, A.; Maden, O.; Saydam, G.S.; Dogan, M.; Ulupinar, H.; Aydin, C.; Topcu, D.I.; Sasmaz, A. Impact of plasma adiponectin levels to the presence and severity of coronary artery disease in patients with metabolic syndrome. Coron. Artery Dis. 2008, 19, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.G.; Haas, M.J.; Mooradian, A.D. The effects of recombinant human growth hormone (rhGH) supplementation on adipokines and C-reactive protein in obese subjects. Growth Horm. IGF Res. 2007, 17, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Makimura, H.; Stanley, T.; Mun, D.; Chen, C.; Wei, J.; Connelly, J.M.; Hemphill, L.C.; Grinspoon, S.K. Reduced growth hormone secretion is associated with increased carotid intima-media thickness in obesity. J. Clin. Endocrinol. Metab. 2009, 94, 5131–5138. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.; Naimo, G.D.; Ricchio, E.; Panno, M.L.; Andò, S. Cross-Talk between Adiponectin and IGF-IR in Breast Cancer. Front Oncol. 2015, 5, 157. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. Obesity-associated Breast Cancer: Analysis of risk factors. Adv. Exp. Med. Biol. 2017, 960, 571–606. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Yamaguchi, T.; Sugimoto, T. Serum insulin-like growth factor-I is negatively associated with serum adiponectin in type 2 diabetes mellitus. Growth Horm. IGF Res. 2011, 21, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Hana, V.; Silha, J.V.; Justova, V.; Lacinova, Z.; Stepan, J.J.; Murphy, L.J. The effects of GH replacement in adult GH-deficient patients: Changes in body composition without concomitant changes in the adipokines and insulin resistance. Clin. Endocrinol. 2004, 60, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Uzum, A.K.; Telci, A.; Alagol, F.; Ozbey, N.C. Serum adipokines and low density lipoprotein subfraction profile in hypopituitary patients with growth hormone deficiency. Pituitary 2012, 15, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Bianda, T.; Zwimpfer, C.; Zapf, J.; Wiesli, P. Changes in insulin sensitivity induced by short-term growth hormone (GH) and insulin-like growth factor I (IGF-I) treatment in GH-deficient adults are not associated with changes in adiponectin levels. Growth Horm. IGF Res. 2005, 15, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.C.; Roberts, C.T., Jr.; Frystyk, J.; Rooney, W.D.; Pollaro, J.R.; Klopfenstein, B.J.; Purnell, J.Q. Short-term, low-dose GH therapy improves insulin sensitivity without modifying cortisol metabolism and ectopic fat accumulation in adults with GH deficiency. J. Clin. Endocrinol. Metab. 2014, 99, E1862–E1869. [Google Scholar] [CrossRef] [PubMed]
- Joaquin, C.; Aguilera, E.; Granada, M.L.; Pastor, M.C.; Salinas, I.; Alonso, N.; Sanmartí, A. Effects of GH treatment in GH-deficient adults on adiponectin, leptin and pregnancy-associated plasma protein-A. Eur. J. Endocrinol. 2008, 158, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, B.L.; Saller, B.; Stratmann, M.; Berg, C.; Mann, K.; Janssen, O.E. Effects of a combination of rhGH and metformin on adiponectin levels in patients with metabolic syndrome. Horm. Metab. Res. 2005, 37, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Zhang, M.; Gao, J.; Zhou, G.Y.; Li, S.Q.; An, Z.M. Study of the correlation between growth hormone deficiency and serum leptin, adiponectin, and visfatin levels in adults. Genet. Mol. Res. 2014, 13, 4050–4056. [Google Scholar] [CrossRef] [PubMed]
- Stawerska, R.; Smyczyńska, J.; Hilczer, M.; Lewiński, A. Relationship between IGF-I Concentration and Metabolic Profile in Children with Growth Hormone Deficiency: The Influence of Children’s Nutritional State as well as the Ghrelin, Leptin, Adiponectin, and Resistin Serum Concentrations. Int. J. Endocrinol. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.; Hizuka, N.; Ishikawa, Y.; Itoh, E.; Yasumoto, K.; Murakami, Y.; Sata, A.; Tsukada, J.; Kurimoto, M.; Okubo, Y.; et al. Serum adiponectin levels in adult growth hormone deficiency and acromegaly. Growth Horm. IGF Res. 2004, 14, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S.; Xu, A.; Tan, K.C.; Wong, L.C.; Tiu, S.C.; Tam, S. Serum adiponectin is reduced in acromegaly and normalized after correction of growth hormone excess. J. Clin. Endocrinol. Metab. 2004, 89, 5448–5453. [Google Scholar] [CrossRef] [PubMed]
- Silha, J.V.; Krsek, M.; Hana, V.; Marek, J.; Jezkova, J.; Weiss, V.; Murphy, L.J. Perturbations in adiponectin, leptin and resistin levels in acromegaly: Lack of correlation with insulin resistance. Clin. Endocrinol. 2003, 58, 736–742. [Google Scholar] [CrossRef]
- Wiesli, P.; Bernays, R.; Brändle, M.; Zwimpfer, C.; Seiler, H.; Zapf, J.; Spinas, G.A.; Schmid, C. Effect of pituitary surgery in patients with acromegaly on adiponectin serum concentrations and alanine aminotransferase activity. Clin. Chim. Acta 2005, 352, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, C.L.; Corbetta, S.; Cappiello, V.; Morpurgo, P.S.; Giavoli, C.; Beck-Peccoz, P.; Arosio, M.; Spada, A. Circulating adiponectin levels and cardiovascular risk factors in acromegalic patients. Eur. J. Endocrinol. 2004, 150, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Olarescu, N.C.; Ueland, T.; Godang, K.; Lindberg-Larsen, R.; Jørgensen, J.O.; Bollerslev, J. Inflammatory adipokines contribute to insulin resistance in active acromegaly and respond differently to different treatment modalities. Eur. J. Endocrinol. 2013, 170, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Olarescu, N.C.; Heck, A.; Godang, K.; Ueland, T.; Bollerslev, J. The Metabolic Risk in Patients Newly Diagnosed with Acromegaly Is Related to Fat Distribution and Circulating Adipokines and Improves after Treatment. Neuroendocrinology 2016, 103, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Olarescu, N.C.; Bollerslev, J. The Impact of Adipose Tissue on Insulin Resistance in Acromegaly. Trends Endocrinol. Metab. 2016, 27, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.; Tsalikian, E.; Beaufrere, B.; Gerich, J.; Haymond, M.; Rizza, R. Insulin resistance in acromegaly: Defects in both hepatic and extrahepatic insulin action. Am. J. Physiol. 1986, 250, E269–E273. [Google Scholar] [PubMed]
- White, U.A.; Maier, J.; Zhao, P.; Richard, A.J.; Stephens, J.M. The modulation of adiponectin by STAT5-activating hormones. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E129–E136. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral adiposity index (VAI): A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Ciresi, A.; Amato, M.C.; Pizzolanti, G.; Giordano Galluzzo, G. Visceral adiposity index is associated with insulin sensitivity and adipocytokine levels in newly diagnosed acromegalic patients. J. Clin. Endocrinol. Metab. 2012, 97, 2907–2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delafontaine, P. Insulin-like growth factor I and its binding proteins in the cardiovascular system. Cardiovasc. Res. 1995, 30, 825–834. [Google Scholar] [CrossRef]
- Colao, A. The GH-IGF-1 axis and the cardiovascular system: Clinical implications. Clin. Endocrinol. 2008, 69, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Auriemma, R.S.; Grasso, L.F.; Pivonello, C.; Simeoli, C.; Patalano, R.; Galdiero, M.; Colao, A. Complications of acromegaly: Cardiovascular, respiratory and metabolic comorbidities. Pituitary 2017, 20, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, M.; Raymond, I.; Kistorp, C.; Hildebrandt, P.; Faber, J.; Kristensen, L.Ø. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur. J. Endocrinol. 2009, 160, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, M.; Kistorp, C.; Raymond, I.; Hildebrandt, P.; Gustafsson, F.; Kristensen, L.Ø.; Faber, J. Plasma insulin-like growth factor I as predictor of progression and all cause mortality in chronic heart failure. Growth Horm. IGF Res. 2009, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Biel, B.; Majda, J.; Szklarska, A.; Lopuszanska, M.; Medras, M.; Anker, S.D.; Banasiak, W.; Poole-Wilson, P.A.; Ponikowski, P. Anabolic deficiency in men with chronic heart failure: Prevalence and detrimental impact on survival. Circulation 2006, 114, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, M. The growth hormone/insulin-like growth factor-I system in chronic heart failure and its interaction with adiponectin. Eur. J. Heart Fail. 2010, 12, 1154–1155. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Quon, M.J.; Kim, J.A.; Koh, K.K. Adiponectin and cardiovascular disease: Response to therapeutic interventions. J. Am. Coll. Cardiol. 2007, 49, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.; Soltys, C.L.; Young, M.E.; Proud, C.G.; Dyck, J.R. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 2004, 279, 32771–32779. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Izumiya, Y.; Sato, K.; Papanicolaou, K.; Kihara, S.; Colucci, W.S.; Sam, F.; Ouchi, N.; Walsh, K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J. Mol. Cell. Cardiol. 2007, 42, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Tamura, T.; Ono, K.; Horiuchi, H.; Kimura, T.; Kita, T.; Furukawa, Y. Insulin-like growth factor axis (insulin-like growth factor-I/insulin-like growth factor-binding protein-3) as a prognostic predictor of heart failure: Association with adiponectin. Eur. J. Heart Fail. 2010, 12, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Juul, A.; Dalgaard, P.; Blum, W.F.; Bang, P.; Hall, K.; Michaelsen, K.F.; Müller, J.; Skakkebaek, N.E. Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: The relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J. Clin. Endocrinol. Metab. 1995, 80, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, A.; Stanca, I.; Copaescu, C.; Martin, S.; Albu, A.; Barbu, C.; Fica, S. Association of serum adiponectin and insulin-like growth factor I levels with parameters of cardiac remodeling in severely obese patients. J. Endocrinol. Investig. 2013, 36, 686–692. [Google Scholar] [CrossRef]
- Katz, L.E.; Gralewski, K.A.; Abrams, P.; Brar, P.C.; Gallagher, P.R.; Lipman, T.H.; Brooks, L.J.; Koren, D. Insulin-like growth factor-I and insulin-like growth factor binding protein-1 are related to cardiovascular disease biomarkers in obese adolescents. Pediatr. Diabetes 2016, 17, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, J.; Velkeniers, B.; Maiter, D.; Haentjens, P.; T’Sjoen, G.; Rietzschel, E.; Corvilain, B.; Abrams, P.; Nobels, F.; Abs, R.; et al. Active acromegaly is associated with decreased hs-CRP and NT-proBNP serum levels: Insights from the Belgian registry of acromegaly. Eur. J. Endocrinol. 2013, 168, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gurbulak, S.; Akin, F.; Yerlikaya, E.; Yaylali, G.F.; Topsakal, S.; Tanriverdi, H.; Akdag, B.; Kaptanoglu, B. Adiponectin and Cardiac Hypertrophy in Acromegaly. Adv. Clin. Exp. Med. 2016, 25, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Roberts, C.T., Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003, 195, 127–137. [Google Scholar] [CrossRef]
- Hursting, S.D.; Digiovanni, J.; Dannenberg, A.J.; Azrad, M.; Leroith, D.; Demark-Wahnefried, W.; Kakarala, M.; Brodie, A.; Berger, N.A. Obesity, energy balance, and cancer: New opportunities for prevention. Cancer Prev. Res. 2012, 5, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, D.Y. Insulin-like growth factor (IGF)-I and IGF binding proteins axis in diabetes mellitus. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF-1), IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Habeeb, B.S.; Kitayama, J.; Nagawa, H. Adiponectin supports cell survival in glucose deprivation through enhancement of autophagic response in colorectal cancer cells. Cancer Sci. 2011, 102, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Chumanevich, A.; Fletcher, E.; Larsen, B.; Lattwein, K.; Kaur, K.; Fayad, R. Adiponectin deficiency: Role in chronic inflammation induced colon cancer. Biochim. Biophys. Acta 2012, 1822, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Baliga, M.S.; Ponemone, V.; Kaur, K.; Larsen, B.; Fletcher, E.; Greene, J.; Fayad, R. Mucus and adiponectin deficiency: Role in chronic inflammation-induced colon cancer. Int. J. Colorectal Dis. 2013, 28, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Wei, E.K.; Giovannucci, E.; Fuchs, C.S.; Willett, W.C.; Mantzoros, C.S. Low plasma adiponectin levels and risk of colorectal cancer in men: A prospective study. J. Natl. Cancer Inst. 2005, 97, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Rzepka-Gorska, I.; Bedner, R.; Cymbaluk-Ploska, A.; Chudecka-Glaz, A. Serum adiponectin in relation to endometrial cancer and endometrial hyperplasia with atypia in obese women. Eur. J. Gynaecol. Oncol. 2008, 29, 594–597. [Google Scholar] [PubMed]
- Tian, Y.F.; Chu, C.H.; Wu, M.H.; Chang, C.L.; Yang, T.; Chou, Y.C.; Hsu, G.C.; Yu, C.P.; Yu, J.C.; Sun, C.A. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocr. Relat. Cancer 2007, 14, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [PubMed]
- Belardi, V.; Gallagher, E.J.; Novosyadlyy, R.; LeRoith, D. Insulin and IGFs in obesity-related breast cancer. J. Mammary Gland Biol. Neoplasia 2013, 18, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Chabrolle, C.; Tosca, L.; Dupont, J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction 2007, 133, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.; Pellegrino, M.; De Amicis, F.; Ricchio, E.; Giordano, F.; Rizza, P.; Catalano, S.; Bonofiglio, D.; Sisci, D.; Panno, M.L.; et al. Evidences that estrogen receptor alpha interferes with adiponectin effects on breast cancer cell growth. Cell Cycle 2014, 13, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, Y.Y.; Yu, B.Y.; Yang, B.S.; Cho, K.H.; Yoon, D.K.; Roh, Y.K. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch. Pharm. Res. 2005, 28, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.; Benaitreau, D.; Dieudonne, M.N.; Leneveu, M.C.; Serazin, V.; Giudicelli, Y.; Pecquery, R. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol. Rep. 2008, 20, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.E.; Nkhata, K.J.; Mizuno, N.K.; Ray, A.; Cleary, M.P. Effects of adiponectin on breast cancer cell growth and signaling. Br. J. Cancer 2008, 98, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Chen, J.Z. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes. Rev. 2009, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Ishihara, S.; Yamaguchi, H.; Murono, K.; Yasuda, K.; Nishikaw, T.; Tanaka, T.; Kiyomatsu, T.; Hata, K.; Kawai, K.; et al. Adiponectin and colorectal cancer. Surg. Today 2017, 47, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Otake, S.; Takeda, H.; Fujishima, S.; Fukui, T.; Orii, T.; Sato, T.; Sasaki, Y.; Nishise, S.; Kawata, S. Decreased levels of plasma adiponectin associated with increased risk of colorectal cancer. World J. Gastroenterol. 2010, 16, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.T.; Xu, Q.; Tong, J.L.; Zhu, M.M.; Huang, M.L.; Ran, Z.H.; Xiao, S.D. Meta-analysis: Circulating adiponectin levels and risk of colorectal cancer and adenoma. J. Dig. Dis. 2011, 12, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Kim, W.J.; Lee, S.A. Association between obesity-related adipokines and colorectal cancer: A case-control study and meta-analysis. World J. Gastroenterol. 2014, 20, 7941–7949. [Google Scholar] [CrossRef] [PubMed]
- Ochs-Balcom, H.M.; Cannioto, R.; Nie, J.; Millen, A.E.; Freudenheim, J.L.; Chen, Z.; Thompson, C.L.; Tracy, R.; Li, L. Adipokines do not mediate the association of obesity and colorectal adenoma. J. Cancer Epidemiol. 2014, 2014, 371254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Vogt, P.K. Class I PI3K in oncogenic cellular transformation. Oncogene 2008, 27, 5486–5496. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.L.; Hofmann, J.N.; Cho, E.; Pollak, M.N.; Chow, W.-H.; Purdue, M.P. Circulating levels of obesity-related markers and risk of renal cell carcinoma in the PLCO cancer screening trial. Cancer Causes Control 2017, 28, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Lewitt, M.S. The role of Growth Hormone/Insulin-like Growth Factor system in visceral adiposity. Biochem. Insights 2017, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orrù, S.; Nigro, E.; Mandola, A.; Alfieri, A.; Buono, P.; Daniele, A.; Mancini, A.; Imperlini, E. A Functional Interplay between IGF-1 and Adiponectin. Int. J. Mol. Sci. 2017, 18, 2145. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102145
Orrù S, Nigro E, Mandola A, Alfieri A, Buono P, Daniele A, Mancini A, Imperlini E. A Functional Interplay between IGF-1 and Adiponectin. International Journal of Molecular Sciences. 2017; 18(10):2145. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102145
Chicago/Turabian StyleOrrù, Stefania, Ersilia Nigro, Annalisa Mandola, Andreina Alfieri, Pasqualina Buono, Aurora Daniele, Annamaria Mancini, and Esther Imperlini. 2017. "A Functional Interplay between IGF-1 and Adiponectin" International Journal of Molecular Sciences 18, no. 10: 2145. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102145