Exploring the Caste-Specific Multi-Layer Defense Mechanism of Formosan Subterranean Termites, Coptotermes formosanus Shiraki
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Volatile Blends of Formosan Subterranean Termites
2.2. Antifungal Activity of Selected Compounds against Entomopathogenic Fungi
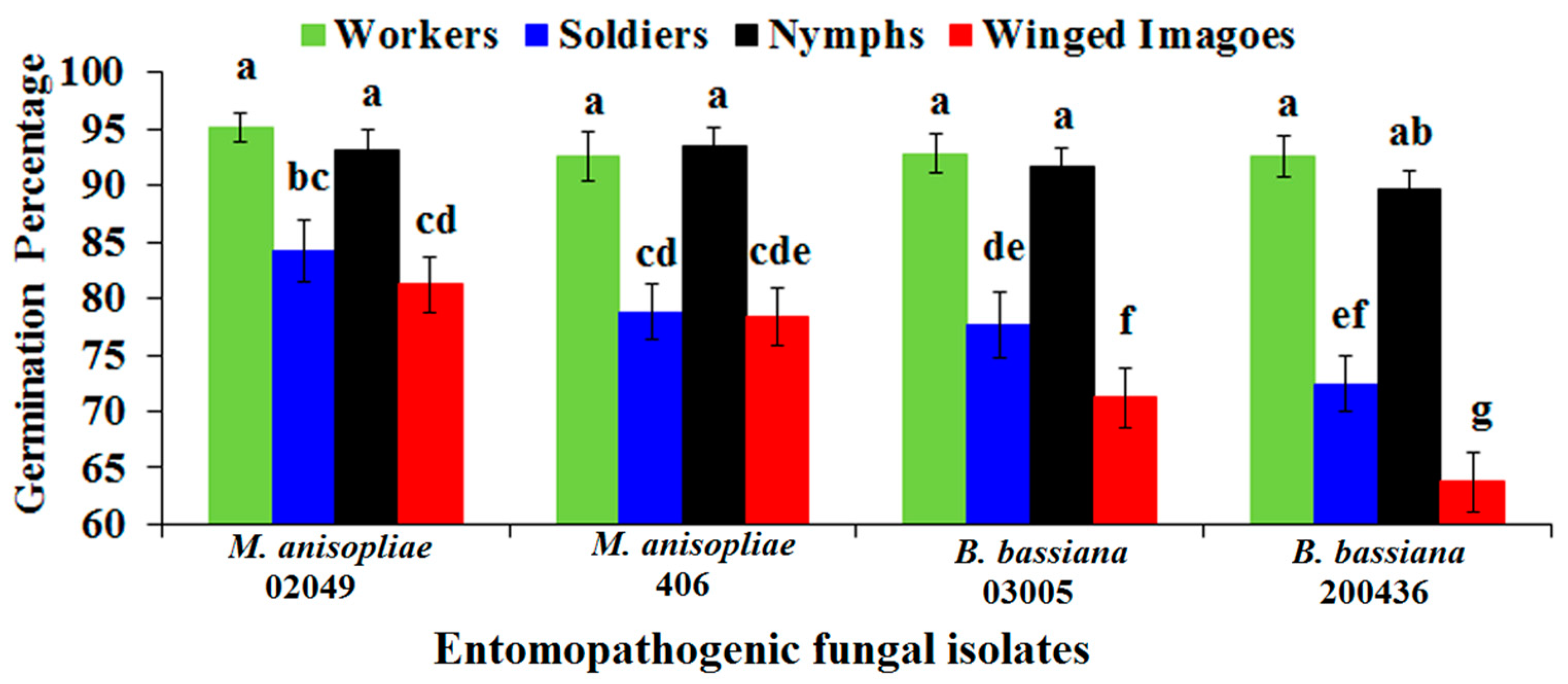
2.3. Conidial Percent Germination of Entomopathogenic Fungi on the Cuticle of Different Castes of FSTs
2.4. Sequence Annotations for Antioxidant Genes of FSTs
2.5. Quantitative Expression Patterns of FSTs Antioxidant Genes
3. Discussion
4. Materials and Methods
4.1. Termite Collection and Maintenance
4.2. Extraction and Analysis of VOCs Released by the Termites
4.3. Origin and Maintenance of Fungal Cultures
4.4. Preparation of Fungal Conidial Suspensions
4.5. Antifungal Assays
4.6. Conidial Germination on the Cuticle of Formosan Subterranean Termites
4.7. Scanning Electron Microscope
4.8. Compilation and Analysis of Antioxidant Sequences of Formosan Subterranean Termites
4.9. Antioxidant Genes Validation and Quantification by qRT-PCR
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HS-SPME | Head space-solid phase micro-extraction |
| VOCs | Volatile organic compounds |
| dp aps | Directly-penetrating appressorium-like structures |
| co | Unipolar-germinated conidium |
| CAT | Catalase |
| DUOX1 | Dual oxidase 1 |
| GRXL | Glutaredoxin-like protein |
| GST | Glutathione S-transferase |
| PRXS | Peroxiredoxin |
| PRXSL | Peroxiredoxin-like protein |
| PRXS1L | Peroxiredoxin 1-like protein |
| Cu/Zn-SOD | Superoxide dismutase Cu/Zn |
| Fe-SOD | Superoxide dismutase Fe |
| TXN | Thioredoxin family protein |
| TXNL | Thioredoxin-like protein |
| TPx | Thioredoxin peroxidase |
| TRx-like-Fd | Thioredoxin-like [2Fe–2S] ferredoxin (Fd) family protein |
| SEM | Scanning electron microscope |
| ESTs | Expressed sequence tags |
References
- Ohkuma, M. Termite symbiotic systems: Efficient bio-recycling of lignocellulose. Appl. Microbiol. Biotechnol. 2003, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Su, N.-Y.; Scheffrahn, R.H. A review of subterranean termite control practices and prospects for Integrated Pest Panagement programmes. Integr. Pest Manag. Rev. 1998, 3, 1–13. [Google Scholar] [CrossRef]
- Kuswanto, E.; Ahmad, I.; Dungani, R. Threat of subterranean termites attack in the Asian countries and their control: A Review. Asian J. Appl. Sci. 2015, 8, 227–239. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y. Germination pattern and inoculum transfer of entomopathogenic fungi and their role in disease resistance among Coptotermes formosanus (Isoptera: Rhinotermitidae). Int. J. Agric. Biol. 2013, 15, 319–324. [Google Scholar]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Bland, J.M.; Gu, W.X. Behavioral and electrophysiological responses of Coptotermes formosanus Shiraki towards entomopathogenic fungal volatiles. Biol. Control 2010, 55, 166–173. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Lei, Y.Y. Differential fluctuation in virulence and VOC profiles among different cultures of entomopathogenic fungi. J. Invertebr. Pathol. 2010, 104, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Li, Y.F.; Cheng, Y.; Liu, Y.; Chen, C.C.; Wen, S.Y. Immune-related transcriptome of Coptotermes formosanus Shiraki workers: The defense mechanism. PLoS ONE 2013, 8, e69543. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Wen, S. Induction of immune response among formosan subterranean termites, Coptotermes formosanus Shiraki (Rhinotermitidae: Isoptera). Afr. J. Microbiol. Res. 2012, 6, 995–1000. [Google Scholar] [CrossRef]
- Chen, J.; Henderson, G.; Grimm, C.; Lloyd, S.; Laine, R. Termites fumigate their nests with naphthalene. Nature 1998, 392, 558–559. [Google Scholar] [CrossRef]
- Wright, M.S.; Lax, A.R.; Henderson, G.; Chen, J. Growth response of Metarhizium anisopliae to two Formosan Subterranean termite nest volatiles, naphthalene and fenchone. Mycologia 2000, 92, 42–45. [Google Scholar] [CrossRef]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochem. Mol. Biol. 2008, 38, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Kodrík, D.; Bednářová, A.; Zemanová, M.; Krishnan, N. Hormonal Regulation of Response to Oxidative Stress in Insects—An Update. Int. J. Mol. Sci. 2015, 16, 25788–25817. [Google Scholar] [CrossRef] [PubMed]
- Rosengaus, R.B.; Guldin, M.R.; Traniello, J.F. Inhibitory effect of termite fecal pellets on fungal spore germination. J. Chem. Ecol. 1998, 24, 1697–1706. [Google Scholar] [CrossRef]
- Rosengaus, R.B.; Lefebvre, M.L.; Traniello, J.F. Inhibition of fungal spore germination by Nasutitermes: Evidence for a possible antiseptic role of soldier defensive secretions. J. Chem. Ecol. 2000, 26, 21–39. [Google Scholar] [CrossRef]
- Howse, P. Sociochemicals of Termites. In Chemical Ecology of Insects; Bell, W.J., Cardé, R.T., Eds.; Chapman & Hall: London, UK, 1984; pp. 475–519. [Google Scholar]
- Chen, J.; Henderson, G.; Grimm, C.C.; Lloyd, S.W.; Laine, R.A. Naphthalene in Formosan Subterranean Termite Carton Nests. J. Agric. Food Chem. 1998, 46, 2337–2339. [Google Scholar] [CrossRef]
- Kaib, M.; Jmhasly, P.; Wilfert, L.; Durka, W.; Franke, S.; Francke, W.; Leuthold, R.H.; Brandl, R. Cuticular hydrocarbons and aggression in the termite Macrotermes Subhyalinus. J. Chem. Ecol. 2004, 30, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Haverty, M.I.; Woodrow, R.J.; Nelson, L.J.; Grace, J.K. Identification of termite species by the Hydrocarbons in their feces. J. Chem. Ecol. 2005, 31, 2119–2151. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Osbrink, W.L.; Cornelius, M.L.; Lax, A.R.; Vigo, C.B. Solid-phase microextraction for the detection of termite cuticular hydrocarbons. J. Chromatogr. A 2001, 932, 119–127. [Google Scholar] [CrossRef]
- Zhukovskaya, M.; Yanagawa, A.; Forschler, B. Grooming behavior as a mechanism of insect disease defense. Insects 2013, 4, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, A.; Imai, T.; Akino, T.; Toh, Y.; Yoshimura, T. Olfactory cues from pathogenic fungus affect the direction of motion of termites, Coptotermes formosanus. J. Chem. Ecol. 2015, 41, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; AlJabr, A. Susceptibility and immune defence mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against entomopathogenic fungal infections. Int. J. Mol. Sci. 2016, 17, 1518. [Google Scholar] [CrossRef] [PubMed]
- Haverty, M.I.; Page, M.; Nelson, L.J.; Blomquist, G.J. Cuticular hydrocarbons of dampwood termites, Zootermopsis: Intra- and intercolony variation and potential as taxonomic characters. J. Chem. Ecol. 1988, 14, 1035–1058. [Google Scholar] [CrossRef] [PubMed]
- Sevala, V.L.; Bagnères, A.-G.; Kuenzli, M.; Blomquist, G.J.; Schal, C. Cuticular hydrocarbons of the Dampwood termite, Zootermopsis nevadensis: Caste differences and role of Lipophorin in transport of hydrocarbons and hydrocarbon metabolites. J. Chem. Ecol. 2000, 26, 765–789. [Google Scholar] [CrossRef]
- Haverty, M.I.; Grace, J.K.; Nelson, L.J.; Yamamoto, R.T. Intercaste, intercolony, and temporal variation in cuticular hydrocarbons of Copotermes formosanus shiraki (Isoptera: Rhinotermitidae). J. Chem. Ecol. 1996, 22, 1813–1834. [Google Scholar] [CrossRef] [PubMed]
- Rosengaus, R.; Traniello, J.; Lefebvre, M.; Maxmen, A. Fungistatic activity of the sternal gland secretion of the dampwood termite Zootermopsis angusticollis. Insectes Soc. 2004, 51, 259–264. [Google Scholar] [CrossRef]
- Wiltz, B.A.; Henderson, G.; Chen, J. Effect of naphthalene, butylated hydroxytoluene, dioctyl phthalate, and adipic dioctyl ester, chemicals found in the nests of the Formosan Subterranean Termite (Isoptera: Rhinotermitidae) on a Saprophytic Mucor sp. (Zygomycetes: Mucorales). Environ. Entomol. 1998, 27, 936–940. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Shelton, T.G. Evidence of cue synergism in termite corpse response behavior. Naturwissenschaften 2012, 99, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Raynor, L.; Mitchell, A.; Walker, R.; Walker, R. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [CrossRef]
- Noirot, C.; Johanna, P.D. Termite Nests: Architecture, regulation and defence. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 121–139. [Google Scholar]
- Kucharski, R.; Maleszka, R. Transcriptional profiling reveals multifunctional roles for transferrin in the honeybee, Apis mellifera. J. Insect Sci. 2003, 3, 1–8. [Google Scholar] [CrossRef]
- Geiser, D.L.; Chavez, C.A.; Flores-Munguia, R.; Winzerling, J.J.; Pham, D.Q.-D. Aedes aegypti ferritin. A cytotoxic protector against iron and oxidative challenge? Eur. J. Biochem. 2003, 270, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Seehuus, S.-C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Gretscher, R.R.; Streicher, P.E.; Strauß, A.S.; Wielsch, N.; Stock, M.; Wang, D.; Boland, W.; Burse, A. A common theme in extracellular fluids of beetles: Extracellular superoxide dismutases crucial for balancing ROS in response to microbial challenge. Sci. Rep. 2016, 6, 24082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Nian, X.; Wu, F.; Shen, Z.; Zhang, B.; Zhang, Q.; Liu, X. Sequence analysis, expression profiles and function of thioredoxin 2 and thioredoxin reductase 1 in resistance to nucleopolyhedrovirus in Helicoverpa armigera. Sci. Rep. 2015, 5, 15531. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; Hutchinson, A.T.; Dalton, J.P.; Donnelly, S. Peroxiredoxin: A central player in immune modulation. Parasite Immunol. 2010, 32, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Ahmed, S.; Al-Jabr, A.M. Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl 2015, 60, 849–859. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ahmed, S. Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Sci. 2009, 16, 511–517. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ruan, L.; Ahmed, S. In vitro and in vivo culturing impacts on the virulence characteristics of serially passed entomopathogenic fungi. J. Food Agric. Environ. 2010, 8, 481–487. [Google Scholar]
- SAS Institute. SAS User’s Guide: Statistics; SAS Institute: Cary, NC, USA, 2000. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Rt (min) | Chemical Formulae | Compounds | RA | |||
|---|---|---|---|---|---|---|
| Winged Imagoes (%) | Nymphs (%) | Workers (%) | Soldiers (%) | |||
| 8.22 | C6H12O2 | n-Hexanoic acid * | 1.35 | — | — | — |
| 11.41 | C9H18O | Nonanal * | — | — | — | 0.54 |
| 12.18 | C9H18O2 | Methyl octanoate * | — | — | 28.17 | 2.70 |
| 13.66 | C10H8 | Naphthalene * | 20.02 | — | — | — |
| 15.95 | C10H20O2 | n-Amyl isovalerate | — | — | 37.53 | 3.39 |
| 16.42 | C10H12O3 | 1-Methoxyethyl benzoate | — | 16.95 | — | — |
| 16.69 | C10H12O | 1-Methoxy-4-(1-propenyl)benzene | — | 2.36 | — | — |
| 16.91 | C10H14O | 2-t-Butylphenol | — | 2.73 | — | — |
| 17.05 | C10H23NS2 | 2-Diisopropylaminoethyl ethyl disulfide | — | 17.98 | — | — |
| 17.30 | C11H22O | n-Undecanal | — | — | 0.69 | 0.10 |
| 17.61 | C11H29 | 2,4,6-Trimethyloctane | — | — | 1.03 | — |
| 17.62 | C12H26 | n-Dodecane | — | — | — | 0.15 |
| 17.81 | C12H18O8 | 1,2,3,4-Butanetetrol, tetraacetate, [R *,S *] | — | — | 3.27 | — |
| 18.24 | C12H24O3 | 2,2-dimethyl-1-(2-hydroxy-1-isopropyl) propyl isobutanoate | — | — | 1.03 | 0.32 |
| 19.22 | C14H28O | Tetradecanal | — | — | 3.17 | 0.30 |
| 19.43 | C14H30 | n-Tetradecane * | 4.10 | — | 5.13 | 0.50 |
| 19.55 | C15H26 | 1,3-Dimethyl-5-n-propyl-adamantane | — | 2.92 | — | — |
| 19.62 | C15H24 | β-Caryophyllene | — | 1.89 | 1.17 | 0.30 |
| 19.95 | C15H24 | Decahydro-1,1,7[1a,a]-trimethyl-4-methylene-1H-cycloprop[e]azulene | — | — | 1.10 | 0.15 |
| 20.42 | C15H32 | 2,6,10-Trimethyldodecane | 4.66 | — | 3.99 | — |
| 20.79 | C15H24O | Butylated hydroxytoluene | 2.25 | — | — | 0.48 |
| 20.92 | C15H32 | n-Pentadecane * | 11.29 | 9.89 | — | 2.31 |
| 21.02 | C15H22 | Cadina-1,2,3-triene | — | — | 2.58 | — |
| 21.80 | C15H32 | 3-Methyltetradecane | 1.28 | — | — | — |
| 22.08 | C15H26O | Cedrol | — | 6.78 | — | — |
| 22.25 | C16H34 | n-Hexadecane * | 15.95 | 3.95 | 2.69 | 0.49 |
| 23.02 | C16H34 | 5-Propyltridecane | 12.54 | — | — | — |
| 23.20 | C16H34 | 3-Methylpentadecane | 1.66 | — | — | — |
| 23.39 | C16H18 | 1,2,3-Trimethyl-4[E]-propenyl-naphthalene | 3.00 | 34.55 | 7.23 | 1.77 |
| 23.79 | C17H36 | n-Heptadecane * | 12.54 | — | — | — |
| 23.96 | C18H38 | 2,6,10-Trimethylpentadecane | 6.38 | — | 1.21 | — |
| 26.10 | C18H38 | n-Octadecane | 2.97 | — | — | — |
| 26.13 | C18H32O2 | [Z,Z]-9,12-Octadecadienoic acid | — | — | — | 12.22 |
| 26.95 | C18H34O2 | Oleic acid * | — | — | — | 65.84 |
| 29.13 | C18H36O2 | Octadecanoic acid | — | — | — | 8.42 |
| No. | Length (bp) | Accession No. | Annotation | Expect Value |
|---|---|---|---|---|
| 1 | 821 | JX876646 | Catalase (CAT) | 5 × 10−111 |
| 2 | 957 | KC571990 | Dual oxidase 1 (DUOX1) | 7 × 10−121 |
| 3 | 847 | KC741176 | Glutaredoxin like protein (GRXL) | 1 × 10−82 |
| 4 | 920 | JX915905 | Glutathione S-transferase (GST) | 2 × 10−139 |
| 5 | 1121 | JX915906 | Peroxiredoxin (PRXS) | 3 × 10−161 |
| 6 | 924 | JX915907 | Peroxiredoxin-like protein (PRXSL) | 3 × 10−105 |
| 7 | 1237 | JX915909 | Peroxiredoxin 1-like protein (PRXS1L) | 5 × 10−137 |
| 8 | 852 | KC741174 | Superoxide Dismutase Cu/Zn (Cu/Zn–SOD1) | 5 × 10−103 |
| 9 | 640 | JX311467 | Superoxide Dismutase Fe (Fe–SOD) | 3 × 10−85 |
| 10 | 909 | JX915904 | Superoxide Dismutase Cu/Zn (Cu/Zn–SOD2) | 4 × 10−75 |
| 11 | 892 | KC632518 | Thioredoxin family protein (TXN1) | 1 × 10−58 |
| 12 | 919 | KC741175 | Thioredoxin family protein (TXN2) | 1 × 10−178 |
| 13 | 910 | JX879125 | Thioredoxin-like protein (TXNL1) | 2 × 10−51 |
| 14 | 917 | KC741177 | Thioredoxin-like protein (TXNL2) | 5 × 10−161 |
| 15 | 849 | JX915908 | Thioredoxin-like protein 4A (TXNL4A) | 4 × 10−60 |
| 16 | 714 | JX879124 | Thioredoxin peroxidase (TPx) | 7 × 10−88 |
| 17 | 889 | KC632520 | Thioredoxin-like [2Fe–2S] ferredoxin (Fd) family protein (TRx-like-Fd) | 5 × 10−132 |
| Genes | Castes | Entomopathogenic Fungi | Castes Statistics | |||
|---|---|---|---|---|---|---|
| M. anisopliae 02049 | M. anisopliae 406 | B. bassiana 03005 | B. bassiana 200436 | |||
| CAT | Workers | 11.15 ± 0.32 b | 7.02 ± 0.49 d | 5.29 ± 0.26 e | 2.98 ± 0.20 g | F = 296.74 |
| Soldiers | 2.96 ± 0.28 g | 2.12 ± 0.15 h | 1.68 ± 0.19 hi | 1.13 ± 0.13 i | df = 3, 32 | |
| Nymphs | 5.05 ± 0.43 e | 3.94 ± 0.19 f | 4.92 ± 0.20 e | 2.09 ± 0.16 h | p < 0.0001 | |
| Winged Imagoes | 15.04 ± 0.48 a | 3.70 ± 0.35 fg | 9.53 ± 0.34 c | 1.86 ± 0.16 hi | ||
| Infection Statistics | F = 344.94; df = 3, 32; p < 0.0001 | F = 78.83 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| DUOX1 | Workers | 1.07 ± 0.14 cde | 0.81 ± 0.07 efgh | 1.21 ± 0.11 cd | 0.59 ± 0.06 hij | F = 24.58 |
| Soldiers | 0.70 ± 0.08 ghi | 0.49 ± 0.11 ij | 0.98 ± 0.09 def | 0.38 ± 0.10 j | df = 3, 32 | |
| Nymphs | 1.81 ± 0.12 a | 0.89 ± 0.09 efg | 1.34 ± 0.11 bc | 0.75 ± 0.05 fghi | p < 0.0001 | |
| Winged Imagoes | 1.59 ± 0.014 ab | 0.83 ± 0.08 efgh | 1.23 ± 0.08 cd | 0.68 ± 0.10 ghi | ||
| Infection Statistics | F = 46.44; df = 3, 32; p < 0.0001 | F = 3.36 | ||||
| df = 9, 32 | ||||||
| p = 0.005 | ||||||
| GRXL | Workers | 2.59 ± 0.16 d | 1.72 ± 0.12 e | 1.82 ± 0.12 e | 1.26 ± 0.13 fg | F = 238.99 |
| Soldiers | 1.57 ± 0.10 ef | 1.06 ± 0.08 g | 1.23 ± 0.10 fg | 0.91 ± 0.07 g | df = 3, 32 | |
| Nymphs | 4.91 ± 0.23 a | 1.69 ± 0.10 e | 3.77 ± 0.15 b | 1.91 ± 0.09 e | p < 0.0001 | |
| Winged Imagoes | 4.63 ± 0.16 a | 3.11 ± 0.10 c | 3.93 ± 0.08 b | 1.09 ± 0.09 g | ||
| Infection Statistics | F = 219.10; df = 3, 32; p < 0.0001 | F = 34.80 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| GST | Workers | 1.95 ± 0.12 ef | 0.91 ± 0.11 h | 1.84 ± 0.09 ef | 0.67 ± 0.08 hi | F = 492.93 |
| Soldiers | 0.88 ± 0.10 hi | 0.71 ± 0.08 hi | 0.70 ± 0.08 hi | 0.61 ± 0.09 i | df = 3, 32 | |
| Nymphs | 3.08 ± 0.11 c | 2.74 ± 0.12 d | 2.11 ± 0.09 e | 1.35 ± 0.10 g | p < 0.0001 | |
| Winged Imagoes | 5.21 ± 0.13 a | 1.69 ± 0.10 f | 3.94 ± 0.10 b | 2.10 ± 0.08 e | ||
| Infection Statistics | F = 202.59; df = 3, 32; p < 0.0001 | F = 57.27 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| PRXS | Workers | 3.88 ± 0.17 a | 1.76 ± 0.10 e | 2.77 ± 0.18 c | 0.83 ± 0.06 gh | F = 114.55 |
| Soldiers | 1.84 ± 0.09 de | 1.20 ± 0.10 f | 1.23 ± 0.11 f | 0.76 ± 0.09 h | df = 3, 32 | |
| Nymphs | 2.54 ± 0.10 c | 1.14 ± 0.09 fg | 2.12 ± 0.10 d | 0.86 ± 0.09 gh | p < 0.0001 | |
| Winged Imagoes | 3.73 ± 0.11 a | 1.85 ± 0.09 de | 3.41 ± 0.11 b | 1.10 ± 0.07 fg | ||
| Infection Statistics | F = 294.23; df = 3, 32; p < 0.0001 | F = 17.60 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| PRXSL | Workers | 2.47 ± 0.12 b | 2.05 ± 0.08 cd | 1.90 ± 0.10 d | 0.96 ± 0.10 ef | F = 65.96 |
| Soldiers | 1.85 ± 0.09 d | 1.23 ± 0.10 e | 1.07 ± 0.08 ef | 0.77 ± 0.06 f | df = 3, 32 | |
| Nymphs | 2.06 ± 0.10 cd | 1.84 ± 0.11 d | 1.17 ± 0.10 e | 0.80 ± 0.08 f | p < 0.0001 | |
| Winged Imagoes | 3.49 ± 0.16 a | 1.77 ± 0.11 d | 2.28 ± 0.13 bc | 1.18 ± 0.08 e | ||
| Infection Statistics | F = 149.75; df = 3, 32; p < 0.0001 | F = 10.27 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| PRXS1L | Workers | 1.01 ± 0.09 cdef | 0.79 ± 0.08 fg | 1.07 ± 0.09 bcde | 0.48 ± 0.09 h | F = 13.70 |
| Soldiers | 1.86 ± 0.10 a | 1.03 ± 0.09 cdef | 0.96 ± 0.08 def | 0.65 ± 0.08 gh | df = 3, 32 | |
| Nymphs | 0.96 ± 0.10 def | 0.86 ± 0.08 efg | 1.11 ± 0.08 bcd | 1.22 ± 0.09 bc | p < 0.0001 | |
| Winged Imagoes | 1.29 ± 0.10 b | 1.19 ± 0.07 bcd | 1.14 ± 0.07 bcd | 1.17 ± 0.08 bcd | ||
| Infection Statistics | F = 16.54; df = 3, 32; p < 0.0001 | F = 11.66 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| Cu/Zn–SOD1 | Workers | 1.10 ± 0.08 fgh | 1.24 ± 0.08 ef | 1.05 ± 0.07 fgh | 0.84 ± 0.07 h | F = 325.13 |
| Soldiers | 1.09 ± 0.08 fgh | 0.88 ± 0.09 gh | 1.21 ± 0.09 ef | 1.10 ± 0.08 fgh | df = 3, 32 | |
| Nymphs | 3.74 ± 0.12 a | 1.44 ± 0.09 e | 2.89 ± 0.12 c | 1.89 ± 0.09 d | p < 0.0001 | |
| Winged Imagoes | 3.17 ± 0.10 b | 2.73 ± 0.10 c | 2.91 ± 0.12 bc | 1.10 ± 0.06 fg | ||
| Infection Statistics | F = 103.32; df = 3, 32; p < 0.0001 | F = 45.14 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| Fe–SOD | Workers | 3.88 ± 0.15 b | 3.16 ± 0.13 d | 3.67 ± 0.11 bc | 2.66 ± 0.10 e | F = 344.02 |
| Soldiers | 1.23 ± 0.08 g | 1.11 ± 0.08 g | 0.92 ± 0.08 gh | 0.62 ± 0.11 h | df = 3, 32 | |
| Nymphs | 4.21 ± 0.12 a | 3.69 ± 0.12 bc | 2.14 ± 0.10 f | 1.11 ± 0.07 g | p < 0.0001 | |
| Winged Imagoes | 3.51 ± 0.13 c | 2.24 ± 0.12 f | 2.94 ± 0.13 de | 2.12 ± 0.10 f | ||
| Infection Statistics | F = 137.33; df = 3, 32; p < 0.0001 | F = 30.98 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| Cu/Zn–SOD2 | Workers | 4.35 ± 0.19 b | 2.96 ± 0.10 c | 1.05 ± 0.09 efg | 0.77 ± 0.09 gh | F = 248.02 |
| Soldiers | 1.02 ± 0.09 efg | 1.21 ± 0.08 e | 0.84 ± 0.09 fgh | 0.52 ± 0.08 h | df = 3, 32 | |
| Nymphs | 2.88 ± 0.12 c | 1.93 ± 0.14 d | 1.66 ± 0.11 d | 1.12 ± 0.07 ef | p < 0.0001 | |
| Winged Imagoes | 5.99 ± 0.16 a | 3.13 ± 0.12 c | 1.12 ± 0.08 ef | 1.92 ± 0.12 d | ||
| Infection Statistics | F = 422.72; df = 3, 32; p < 0.0001 | F = 68.00 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| TXN1 | Workers | 2.74 ± 0.12 ab | 2.06 ± 0.11 de | 1.73 ± 0.12 fg | 1.13 ± 0.08 i | F = 32.14 |
| Soldiers | 1.82 ± 0.17 efg | 1.55 ± 0.12 gh | 1.12 ± 0.09 ij | 0.81 ± 0.07 j | df = 3, 32 | |
| Nymphs | 2.47 ± 0.10 bc | 1.92 ± 0.10 def | 2.18 ± 0.12 cd | 1.27 ± 0.10 hi | p < 0.0001 | |
| Winged Imagoes | 2.96 ± 0.11 a | 1.76 ± 0.10 efg | 1.94 ± 0.12 def | 1.14 ± 0.07 i | ||
| Infection Statistics | F = 111.59; df = 3, 32; p < 0.0001 | F = 4.34 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| TXN2 | Workers | 2.13 ± 0.11 c | 1.89 ± 0.13 cde | 0.87 ± 0.10 g | 0.86 ± 0.09 g | F = 24.49 |
| Soldiers | 1.94 ± 0.11 cd | 1.48 ± 0.11 f | 1.13 ± 0.06 g | 0.93 ± 0.11 g | df = 3, 32 | |
| Nymphs | 2.76 ± 0.13 b | 1.73 ± 0.13 def | 1.91 ± 0.12 cde | 1.09 ± 0.08 g | p < 0.0001 | |
| Winged Imagoes | 3.75 ± 0.14 a | 1.17 ± 0.06 g | 1.60 ± 0.11 ef | 0.92 ± 0.12 g | ||
| Infection Statistics | F = 176.34; df = 3, 32; p < 0.0001 | F = 19.92 | ||||
| df = 9, 32 | ||||||
| p = 0.0009 | ||||||
| TXNL1 | Workers | 1.76 ± 0.14 ab | 0.81 ± 0.09 e | 1.38 ± 0.11 cd | 0.21 ± 0.05 g | F = 0.46 |
| Soldiers | 1.91 ± 0.11 a | 0.65 ± 0.09 ef | 1.17 ± 0.12 d | 0.47 ± 0.09 fg | df = 3, 32 | |
| Nymphs | 1.50 ± 0.10 bc | 1.16 ± 0.07 d | 1.29 ± 0.11 cd | 0.48 ± 0.08 fg | p > 0.05 | |
| Winged Imagoes | 1.93 ± 0.12 a | 1.15 ± 0.11 d | 0.84 ± 0.08 e | 0.26 ± 0.08 g | ||
| Infection statistics | F = 142.88; df = 3, 32; p < 0.0001 | F = 6.06 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| TXNL2 | Workers | 2.11 ± 0.11 b | 1.18 ± 0.11 de | 1.86 ± 0.11 bc | 0.68 ± 0.09 f | F = 15.95 |
| Soldiers | 1.96 ± 0.14 bc | 1.13 ± 0.06 de | 1.72 ± 0.11 c | 0.79 ± 0.09 f | df = 3, 32 | |
| Nymphs | 2.17 ± 0.13 b | 1.67 ± 0.12 c | 0.93 ± 0.07 ef | 0.65 ± 0.12 f | p < 0.0001 | |
| Winged Imagoes | 2.79 ± 0.15 a | 1.16 ± 0.07 de | 2.17 ± 0.11 b | 1.26 ± 0.14 d | ||
| Infection Statistics | F = 115.31; df = 3, 32; p < 0.0001 | F = 9.56 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| TXNL4A | Workers | 4.73 ± 0.15 b | 2.82 ± 0.14 ef | 3.46 ± 0.16 d | 1.13 ± 0.08 j | F = 159.16 |
| Soldiers | 2.09 ± 0.14 gh | 1.53 ± 0.12 i | 1.91 ± 0.14 hi | 1.07 ± 0.07 j | df = 3, 32 | |
| Nymphs | 3.86 ± 0.14 c | 2.30 ± 0.12 g | 3.20 ± 0.13 de | 1.94 ± 0.13 gh | p < 0.0001 | |
| Winged Imagoes | 5.31 ± 0.18 a | 2.72 ± 0.13 f | 4.76 ± 0.16 b | 2.13 ± 0.12 gh | ||
| Infection Statistics | F = 253.32; df = 3, 32; p < 0.0001 | F = 20.22 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| TPx | Workers | 3.74 ± 0.15 ab | 2.46 ± 0.10 g | 3.35 ± 0.15 cd | 1.73 ± 0.12 h | F = 142.07 |
| Soldiers | 1.88 ± 0.13 h | 1.12 ± 0.09 i | 1.21 ± 0.10 i | 0.68 ± 0.12 j | df = 3, 32 | |
| Nymphs | 3.47 ± 0.16 bc | 1.94 ± 0.13 h | 2.69 ± 0.14 gh | 1.12 ± 0.08 i | p < 0.0001 | |
| Winged Imagoes | 3.95 ± 0.14 a | 3.09 ± 0.12 de | 2.93 ± 0.14 ef | 1.08 ± 0.08 i | ||
| Infection Statistics | F = 201.29; df = 3, 32; p < 0.0001 | F = 9.66 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| TRx-like-Fd | Workers | 3.53 ± 0.16 a | 2.82 ± 0.17 b | 3.05 ± 0.14 b | 1.65 ± 0.12 d | F = 84.27 |
| Soldiers | 1.82 ± 0.15 cd | 1.26 ± 0.13 e | 1.12 ± 0.07 ef | 0.73 ± 0.09 g | df = 3, 32 | |
| Nymphs | 2.79 ± 0.16 b | 2.14 ± 0.10 c | 1.68 ± 0.13 d | 0.76 ± 0.09 fg | p < 0.0001 | |
| Winged Imagoes | 3.07 ± 0.11 b | 2.18 ± 0.14 c | 2.19 ± 0.12 c | 1.25 ± 0.13 e | ||
| Infection Statistics | F = 117.62; df = 3, 32; p < 0.0001 | F = 7.84 | ||||
| df = 9, 32 | ||||||
| p < 0.0001 | ||||||
| Gene | Product Length | Accession Number | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|---|
| Catalase (CAT) | 97 bp | JX876646 | TGGCACCAACTACCTTCAAA | TCCTTGGTGATGCATTGTTT |
| Dual oxidase 1 (DUOX1) | 95 bp | KC571990 | AGTTTGACCTCAGGACCATCC | AGTGACTGCTTTCAGCCCAG |
| Glutaredoxin like protein (GRXL) | 87 bp | KC741176 | GGAATTGCATCCAGTAGGGAGA | CTGCTCCCTTGCCTCTTCTT |
| Glutathione S-transferase (GST) | 99 bp | JX915905 | GTATTGGCAGGTTCGTGTTG | TACAGCCTCAGCTTCCCTTT |
| Peroxiredoxin (PRXS) | 106 bp | JX915906 | ACCTGTTGGTCGCAGTGTAG | TTCTTGTCCTGGCTTCCAGC |
| Peroxiredoxin-like protein (PRXSL) | 112 bp | JX915907 | TTCATGCTGTAGGGCGTGTT | TCACAGAGCTCCACACTTGG |
| Peroxiredoxin 1-like protein (PRXS1L) | 97 bp | JX915909 | GCATATTCTGACCGTGCAGC | TGTTGACCCAAGCAAGGTGA |
| Superoxide Dismutase Cu/Zn (Cu/Zn-SOD1) | 93 bp | KC741174 | GTAACGGGGGAAGTGACTGG | TCCAGCACTTGTACAGCCAT |
| Superoxide Dismutase Fe (Fe-SOD) | 127 bp | JX311467 | ACAGTTGATGCTTGGGAACA | TGAGACCAGCAGCCTTAAAC |
| Superoxide Dismutase Cu/Zn (Cu/Zn-SOD2) | 90 bp | JX915904 | AATGGAGAAGTGGTCAAGGG | TGACAGACCAGTCACTTCCC |
| Thioredoxin family protein (TXN1) | 81 bp | KC632518 | CTTACACCGGAGGACGACAT | AACCATCGGGTGCTCGATTC |
| Thioredoxin family protein (TXN2) | 75 bp | KC741175 | TCTGAATGTTGCCCGTGTGA | GGCACTTCCCAGACTCCAAA |
| Thioredoxin-like protein (TXNL1) | 97 bp | JX879125 | ACAAAGCTTACGGAGGCAGG | GCTCCTCAATTCTGGGTGCT |
| Thioredoxin-like protein (TXNL2) | 81 bp | KC741177 | GGCCACACCACAACTAAAGC | ATGCAGGTGTTTTGGTCGGA |
| Thioredoxin-like protein 4A (TXNL4A) | 80 bp | JX915908 | TGGCCATTGGAAGACAAGCA | ACTAGACCTCGACCTTTGCG |
| Thioredoxin peroxidase (TPx) | 80 bp | JX879124 | TGCCGCAAAACATGGAGAAG | TGCTCTCATTCGGCTTAGGA |
| Thioredoxin-like [2Fe–2S] ferredoxin (Fd) family protein (TRx-like-Fd) | 106 bp | KC632520 | CTTGTGTCAACGCCCCAATG | GGCACTCGGCCATTTTTCAA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; Wen, S.-Y.; Tian, M.-Y. Exploring the Caste-Specific Multi-Layer Defense Mechanism of Formosan Subterranean Termites, Coptotermes formosanus Shiraki. Int. J. Mol. Sci. 2017, 18, 2694. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122694
Hussain A, Wen S-Y, Tian M-Y. Exploring the Caste-Specific Multi-Layer Defense Mechanism of Formosan Subterranean Termites, Coptotermes formosanus Shiraki. International Journal of Molecular Sciences. 2017; 18(12):2694. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122694
Chicago/Turabian StyleHussain, Abid, Shuo-Yang Wen, and Ming-Yi Tian. 2017. "Exploring the Caste-Specific Multi-Layer Defense Mechanism of Formosan Subterranean Termites, Coptotermes formosanus Shiraki" International Journal of Molecular Sciences 18, no. 12: 2694. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122694





