Melatonin Inhibits Androgen Receptor Splice Variant-7 (AR-V7)-Induced Nuclear Factor-Kappa B (NF-κB) Activation and NF-κB Activator-Induced AR-V7 Expression in Prostate Cancer Cells: Potential Implications for the Use of Melatonin in Castration-Resistant Prostate Cancer (CRPC) Therapy
Abstract
:1. Introduction
2. Results
2.1. Visualization of Overexpressed Enhanced Green Fluorescent Protein (EGFP)-Tagged AR and AR-V7 in Prostate Cancer Cells
2.2. Expression of AR and Its Variants (AR-Vs) in Prostate Cancer Cells
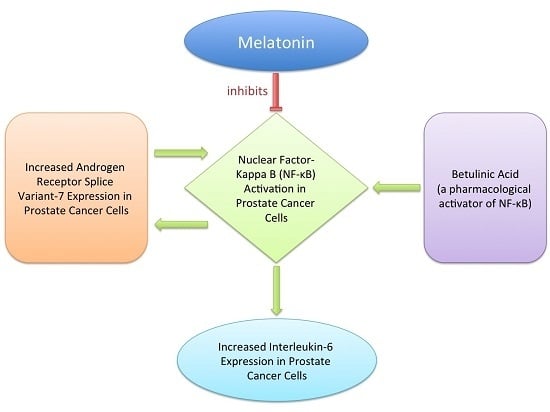
2.3. Effects of AR-V7 on NF-κB Activity
2.4. Inhibition of AR-V7 Induced IL-6 Gene Expression by Melatonin
2.5. Inhibition of NF-κB Induced AR-V7 Expression by Melatonin
2.6. Involvement of Membrane MT1 Receptor in Melatonin’s Inhibitory Effect on AR-V7-Induced NF-κB Activation
3. Discussion
4. Materials and Methods
4.1. Cells, Plasmids, and Chemicals
4.2. Fluorescence Microscopy
4.3. Immunoblot Analysis
4.4. Luciferase Reporter Assays
4.5. Quantitative Polymerase Chain Reaction (Q-PCR)
4.6. Statistical and Data Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]; International Agency for Research on Cancer: Lyon, France, 2013; Available online: http://globocan.iarc.fr (accessed on 22 March 2017).
- Chen, Y.; Clegg, N.J.; Scher, H.I. Anti-androgens and androgen depleting therapies in prostate cancer: New agents for an established target. Lancet Oncol. 2009, 10, 981–991. [Google Scholar] [CrossRef]
- Attard, G.; Reid, A.H.; A’Hern, R.; Parker, C.; Oommen, N.B.; Folkerd, E.; Messiou, C.; Molife, L.R.; Maier, G.; Thompson, E.; et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 2009, 27, 3742–3748. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [PubMed]
- Guo, Z.; Yang, X.; Sun, F.; Jiang, R.; Linn, D.E.; Chen, H.; Kong, X.; Melamed, J.; Tepper, C.G.; Kung, H.J.; et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen-depletion-resistant growth. Cancer Res. 2009, 69, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Dunn, T.A.; Wei, S.; Isharwal, S.; Veltri, R.W.; Humphreys, E.; Han, M.; Partin, A.W.; Vessella, R.L.; Isaacs, W.B.; et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009, 69, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sprenger, C.C.; Vessella, R.L.; Haugk, K.; Soriano, K.; Mostaghell, E.A.; Page, S.T.; Coleman, I.M.; Nguyen, H.M.; Sun, H.; et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J. Clin. Investig. 2010, 120, 2715–2730. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Isaacs, W.B.; Luo, J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate 2011, 71, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Denmeade, S.R.; Luo, J. Molecular processes leading to aberrant androgen receptor signaling and castration resistance in prostate cancer. Expert Rev. Endocrinol. Metab. 2010, 5, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Hornberg, E.; Ylitalo, E.B.; Crnalic, S.; Antti, H.; Stattin, P.; Widmark, A.; Bergh, A.; Wikstrom, P. Expression of androgen receptor splice variants in prostate cancer bone metastasis is associated with castration-resistance and short survival. PLoS ONE 2011, 6, e19059. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lu, C.; Mostaghel, E.A.; Yegnasubramanian, S.; Gurel, M.; Tannahill, C.; Edwards, J.; Isaacs, W.B.; Nelson, P.S.; Bluemn, E.; et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012, 72, 3457–3462. [Google Scholar] [CrossRef] [PubMed]
- Mostaghel, E.A.; Marck, B.T.; Plymate, S.R.; Vessella, R.L.; Balk, S.; Matsumoto, A.M.; Nelson, P.S.; Montgomery, R.B. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 2011, 17, 5913–5925. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 317, 1028–1038. [Google Scholar]
- Xi, S.C.; Tam, P.C.; Brown, G.M.; Pang, S.F.; Shiu, S.Y.W. Potential involvement of MT1 receptor and attenuated sex-steroid-induced calcium influx in the direct anti-proliferative action of melatonin on androgen-responsive LNCaP human prostate cancer cells. J. Pineal Res. 2000, 29, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.C.; Siu, S.W.F.; Fong, S.W.; Shiu, S.Y.W. Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: Association of antiproliferative action of the pineal hormone with MT1 receptor protein expression. Prostate 2001, 46, 52–61. [Google Scholar] [CrossRef]
- Siu, S.W.F.; Lau, K.W.; Tam, P.C.; Shiu, S.Y.W. Melatonin and prostate cancer cell proliferation: Interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate 2002, 52, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.Y.W.; Law, I.C.; Lau, K.W.; Tam, P.C.; Yip, A.W.C.; Ng, W.T. Melatonin slowed the early biochemical progression of hormone-refractory prostate cancer in a patient whose prostate tumor tissue expressed MT1 receptor subtype. J. Pineal Res. 2003, 35, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.W.; Mo, C.W.; Yao, K.M.; Shiu, S.Y.W. Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22Rv1 human prostate cancer cells: Implications for prostate cancer chemoprevention. J. Pineal Res. 2007, 42, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.Y.W.; Pang, B.; Tam, C.W.; Yao, K.M. Signal transduction of receptor-mediated antiproliferative action of melatonin on human prostate epithelial cells involves dual activation of Gαs and Gαq proteins. J. Pineal Res. 2010, 49, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.W.; Shiu, S.Y.W. Functional interplay between melatonin receptor-mediated antiproliferative signaling and androgen receptor signaling in human prostate epithelial cells: Potential implications for therapeutic strategies against prostate cancer. J. Pineal Res. 2011, 51, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.Y.W.; Leung, W.Y.; Tam, C.W.; Liu, V.W.S.; Yao, K.M. Melatonin MT1 receptor-induced transcriptional up-regulation of p27Kip1 in prostate cancer antiproliferation is mediated via inhibition of constitutively active nuclear factor kappa B (NF-κB): Potential implications on prostate cancer chemoprevention and therapy. J. Pineal Res. 2013, 54, 69–79. [Google Scholar] [PubMed]
- Suh, J.; Rabson, A.B. NF-κB activation in human prostate cancer: Important mediator or epiphenomenon? J. Cell. Biochem. 2004, 91, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Cronauer, M.V.; Schrader, M.; Möller, P.; Marienfeld, R.B. NF-κB signaling in prostate cancer: A promising therapeutic target? World J. Urol. 2012, 30, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Cooper, C.S.; de Bono, J.S. Steroid hormone receptors in prostate cancer: A hard habit to break? Cancer Cell 2009, 16, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sawyers, C.L.; Scher, H.I. Targeting the androgen receptor pathway in prostate cancer. Curr. Opin. Pharmacol. 2008, 8, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Paule, B.; Terry, S.; Kheuang, L.; Soyeux, P.; Vacherot, F.; de la Taille, A. The NF-κB/IL-6 pathway in metastatic androgen-independent prostate cancer: New therapeutic approaches? World J. Urol. 2007, 25, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yamashita, H.; Yu, X.; Wang, J.; Franco, O.E.; Wang, Y.; Hayward, S.W.; Matusik, R.J. Inhibition of NF-κB signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene 2015, 34, 3700–3710. [Google Scholar] [CrossRef] [PubMed]
- Giri, D.; Ozen, M.; Ittmann, M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am. J. Pathol. 2001, 159, 2159–2165. [Google Scholar] [CrossRef]
- Lee, S.O.; Lou, W.; Johnson, C.S.; Trump, D.L.; Gao, A.C. Interleukin-6 protects LNCaP cells from apoptosis induced by androgen deprivation through the Stat3 pathway. Prostate 2004, 60, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Andrews, B.; Kattan, M.W.; Kim, J.; Wheeler, T.M.; Slawin, K.M. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 2001, 58, 1008–1015. [Google Scholar] [CrossRef]
- Drachenberg, D.E.; Elgamal, A.A.; Rowbotham, R.; Peterson, M.; Murphy, G.P. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate 1999, 41, 127–133. [Google Scholar] [CrossRef]
- Austin, D.C.; Strand, D.W.; Love, H.L.; Franco, O.E.; Jang, A.; Grabowska, M.M.; Miller, N.L.; Hameed, O.; Clark, P.E.; Fowke, J.H.; et al. NF-κB and androgen receptor variant expression correlate with human BPH progression. Prostate 2016, 76, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P.; Gao, A.C. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef] [PubMed]
- Seely, D.; Wu, P.; Fritz, H.; Kennedy, D.A.; Tsui, T.; Seely, A.J.; Mills, E. Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. Integr. Cancer Ther. 2012, 11, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Lapouge, G.; Erdmann, E.; Marcias, G.; Jagla, M.; Monge, A.; Kessler, P.; Serra, S.; Lang, H.; Jacqmin, D.; Bergerat, J.P.; Céraline, J. Unexpected paracrine action of prostate cancer cells harboring a new class of androgen receptor mutation—A new paradigm for cooperation among prostate tumor cells. Int. J. Cancer 2007, 121, 1238–1244. [Google Scholar] [CrossRef] [PubMed]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, V.W.S.; Yau, W.L.; Tam, C.W.; Yao, K.-M.; Shiu, S.Y.W. Melatonin Inhibits Androgen Receptor Splice Variant-7 (AR-V7)-Induced Nuclear Factor-Kappa B (NF-κB) Activation and NF-κB Activator-Induced AR-V7 Expression in Prostate Cancer Cells: Potential Implications for the Use of Melatonin in Castration-Resistant Prostate Cancer (CRPC) Therapy. Int. J. Mol. Sci. 2017, 18, 1130. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061130
Liu VWS, Yau WL, Tam CW, Yao K-M, Shiu SYW. Melatonin Inhibits Androgen Receptor Splice Variant-7 (AR-V7)-Induced Nuclear Factor-Kappa B (NF-κB) Activation and NF-κB Activator-Induced AR-V7 Expression in Prostate Cancer Cells: Potential Implications for the Use of Melatonin in Castration-Resistant Prostate Cancer (CRPC) Therapy. International Journal of Molecular Sciences. 2017; 18(6):1130. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061130
Chicago/Turabian StyleLiu, Vincent Wing Sun, Wing Lung Yau, Chun Wai Tam, Kwok-Ming Yao, and Stephen Yuen Wing Shiu. 2017. "Melatonin Inhibits Androgen Receptor Splice Variant-7 (AR-V7)-Induced Nuclear Factor-Kappa B (NF-κB) Activation and NF-κB Activator-Induced AR-V7 Expression in Prostate Cancer Cells: Potential Implications for the Use of Melatonin in Castration-Resistant Prostate Cancer (CRPC) Therapy" International Journal of Molecular Sciences 18, no. 6: 1130. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061130