Acute Effect of Metformin on Postprandial Hypertriglyceridemia through Delayed Gastric Emptying
Abstract
:1. Introduction
2. Results
2.1. Metformin Treatment for One Week Decreased Postprandial Plasma Glucose Levels without Loss of Body Weight
2.2. Metformin Treatment for One Week Decreased Postprandial Plasma TG
2.3. Both Single and Long-Term Metformin Treatments Decreased Postprandial Plasma TG Levels
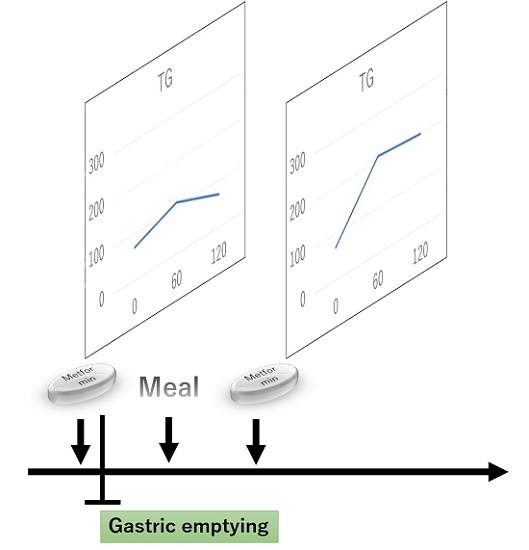
2.4. A Single Metformin Pre-Treatment Decreased Postprandial Plasma TG Levels Compared with Post-Treatment
2.5. Effect of Metformin on Gastric Size during Olive Oil-Loading
2.6. Metformin Did Not Affect Intestinal Lipid Absorption
2.7. The Metformin Treatment Group Had Lower Rates of Whole Body Fatty Acid Oxidation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Pharmacological Intervention
4.4. Body Weight and Food Intake
4.5. Oral Glucose Tolerance Test (OGTT)
4.6. Oral Lipid Tolerance Test (OLTT)
4.7. ApoB-48 Secretion from Primary Small Intestinal Enterocytes
4.8. Analysis of the Actual Ratio of 13CO2 to 12CO2 in Sampled Gas
4.9. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R. Diabetic dyslipidaemia: From basic research to clinical practice. Diabetologia 2003, 46, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Maeda, E.; Yoshino, G.; Kasuga, M. Diabetes mellitus as a risk factor for arteriosclerosis. Nihon Rinsho 1993, 51, 2170–2176. [Google Scholar] [PubMed]
- Suryabhan, L.L.; Chandrashekhar, M.I.; Ratnendra, R.S.; Prerna, D.N. A comparative study on the fasting and the postprandial dyslipidaemia in type 2 diabetes mellitus. J. Clin. Diagn. Res. 2013, 7, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Mero, N.; Malmström, R.; Steiner, G.; Taskinen, M.R.; Syvänne, M. Postprandial metabolism of apolipoprotein B-48- and B-100-containing particles in type 2 diabetes mellitus: Relations to angiographically verified severity of coronary artery disease. Atherosclerosis 2000, 150, 167–177. [Google Scholar] [CrossRef]
- Karpe, F.; Steiner, G.; Uffelman, K.; Olivecrona, T.; Hamsten, a. postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis 1994, 106, 83–97. [Google Scholar] [CrossRef]
- Krauss, R.M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004, 27, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Goodman, A.M. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N. Engl. J. Med. 1995, 333, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Zhou, M.Y.; Chen, Y.D.; Reaven, G.M. Effect of metformin on postprandial lipemia in patients with fairly to poorly controlled NIDDM. Diabetes Care 1994, 17, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Gerrard, J.; Dalla, M.C.; Firbank, M.J.; Lane, A.; English, P.T.; Cobelli, C.; Taylor, R. Pioglitazone decreases fasting and postprandial endogenous glucose production in proportion to decrease in hepatic triglyceride content. Diabetes 2008, 57, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. Effects of pioglitazone on lipid and lipoprotein metabolism. Diabetes Obes. Metab. 2007, 9, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Spanheimer, R.; Betteridge, D.J.; Tan, M.H.; Ferrannini, E.; Charbonnel, B.; Investigators, P. Long-term lipid effects of pioglitazone by baseline anti-hyperglycemia medication therapy and statin use from the PROactive experience (PROactive 14). Am. J. Cardiol. 2009, 104, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Pruski, M.; Krysiak, R.; Okopien, B. Pleiotropic action of short-term metformin and fenofibrate treatment, combined with lifestyle intervention, in type 2 diabetic patients with mixed dyslipidemia. Diabetes Care 2009, 32, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Samadfam, R.; Awori, M.; Bénardeau, A.; Bauss, F.; Sebokova, E.; Wright, M.; Smith, S.Y. Combination treatment with pioglitazone and fenofibrate attenuates pioglitazone-mediated acceleration of bone loss in ovariectomized rats. J. Endocrinol. 2012, 212, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Groop, L.; Widén, E.; Franssila-Kallunki, A.; Ekstrand, A.; Saloranta, C.; Schalin, C.; Eriksson, J. Different effects of insulin and oral antidiabetic agents on glucose and energy metabolism in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1989, 32, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Rains, S.G.; Wilson, G.A.; Richmond, W.; Elkeles, R.S. The effect of glibenclamide and metformin on serum lipoproteins in type 2 diabetes. Diabet. Med. 1988, 5, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Mourão-Júnior, C.A.; Sá, J.R.; Guedes, O.M.; Dib, S.A. Effects of metformin on the glycemic control, lipid profile, and arterial blood pressure of type 2 diabetic patients with metabolic syndrome already on insulin. Braz. J. Med. Biol. Res. 2006, 39, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhu, S.; Zhang, X.; Guo, Y.; Shi, Y.; Chen, Z.; Leung, S.W. Comparative efficacy of glimepiride and metformin in monotherapy of type 2 diabetes mellitus: Meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2013, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Panarotto, D.; Rémillard, P.; Bouffard, L.; Maheux, P. Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue-specific manner. Eur. J. Clin. Investig. 2002, 32, 84–92. [Google Scholar] [CrossRef]
- Eckel, R.H.; Yost, T.J. Weight reduction increases adipose tissue lipoprotein lipase responsiveness in obese women. J. Clin. Investig. 1987, 80, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.S.; Brunzell, J.D. Increase of adipose tissue lipoprotein lipase activity after weight loss. J. Clin. Investig. 1981, 67, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.J. The effects of high- and medium-dose metformin therapy on cardiovascular risk factors in patients with type II diabetes. Diabetes Care 1996, 19, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Clinical pharmacokinetics of metformin. Clin. Pharmacokin. 1996, 30, 359–371. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Tanaka, M.; Okada, H.; Mistuhashi, K.; Kimura, T.; Kitagawa, N.; Fukuda, T.; Majima, S.; Fukuda, Y.; Tanaka, Y.; et al. Postprandial hyperglycemia was ameliorated by taking metformin 30 min before a meal than taking metformin with a meal; a randomized, open-label, crossover pilot study. Endocrine 2016, 52, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.K.; Deane, A.M.; Jones, K.L.; Rayner, C.K.; Horowitz, M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat. Rev. Endocrinol. 2015, 11, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Gonlachanvit, S.; Hsu, C.W.; Boden, G.H.; Knight, L.C.; Maurer, A.H.; Fisher, R.S.; Parkman, H.P. Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type-II diabetic patients. Dig. Dis. Sci. 2003, 48, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Reeds, D.N.; Crimmins, D.L.; Patterson, B.W.; Laciny, E.; Wang, S.; Tran, H.D.; Griest, T.A.; Rometo, D.A.; Dunai, J.; et al. Xenin-25 delays gastric emptying and reduces postprandial glucose levels in humans with and without type 2 diabetes. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G301–G309. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.T.; Casey, C.; Phillips, P.J.; Connor, H.; Ward, J.D.; Woods, H.F. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br. J. Clin. Pharmacol. 1981, 12, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, C.; Bailey, C.J. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 1994, 24, 49–57. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Aslam, M.; Aggarwal, S.; Sharma, K.K.; Galav, V.; Madhu, S.V. Postprandial hypertriglyceridemia predicts development of insulin resistance glucose intolerance and type 2 diabetes. PLoS ONE 2016, 11, e0145730. [Google Scholar] [CrossRef] [PubMed]
- Excoffon, K.J.; Liu, G.; Miao, L.; Wilson, J.E.; McManus, B.M.; Semenkovich, C.F.; Coleman, T.; Benoit, P.; Duverger, N.; Branellec, D.; et al. Correction of hypertriglyceridemia and impaired fat tolerance in lipoprotein lipase-deficient mice by adenovirus-mediated expression of human lipoprotein lipase. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.; Havel, P.J.; Sniderman, A.D.; Cianflone, K. Reduced body weight, adipose tissue, and leptin levels despite increased energy intake in female mice lacking acylation-stimulating protein. Endocrinology 2000, 141, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Ushio, M.; Nishio, Y.; Sekine, O.; Nagai, Y.; Maeno, Y.; Ugi, S.; Yoshizaki, T.; Morino, K.; Kume, S.; Kashiwagi, A.; et al. Ezetimibe prevents hepatic steatosis induced by a high-fat but not a high-fructose diet. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E293–E304. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Gotoh, N.; Kubo, A.; Kanda, J.; Nagai, T.; Mizobe, H.; Yoshinaga, K.; Kojima, K.; Watanabe, H.; Wada, S. Comparison of catabolism rate of fatty acids to carbon dioxide in mice. Eur. J. Lipid Sci. Technol. 2012, 114, 1340–1344. [Google Scholar] [CrossRef]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, D.; Morino, K.; Nakagawa, F.; Murata, K.; Sekine, O.; Beppu, F.; Gotoh, N.; Ugi, S.; Maegawa, H. Acute Effect of Metformin on Postprandial Hypertriglyceridemia through Delayed Gastric Emptying. Int. J. Mol. Sci. 2017, 18, 1282. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061282
Sato D, Morino K, Nakagawa F, Murata K, Sekine O, Beppu F, Gotoh N, Ugi S, Maegawa H. Acute Effect of Metformin on Postprandial Hypertriglyceridemia through Delayed Gastric Emptying. International Journal of Molecular Sciences. 2017; 18(6):1282. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061282
Chicago/Turabian StyleSato, Daisuke, Katsutaro Morino, Fumiyuki Nakagawa, Koichiro Murata, Osamu Sekine, Fumiaki Beppu, Naohiro Gotoh, Satoshi Ugi, and Hiroshi Maegawa. 2017. "Acute Effect of Metformin on Postprandial Hypertriglyceridemia through Delayed Gastric Emptying" International Journal of Molecular Sciences 18, no. 6: 1282. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061282