The Application of Heptamethine Cyanine Dye DZ-1 and Indocyanine Green for Imaging and Targeting in Xenograft Models of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
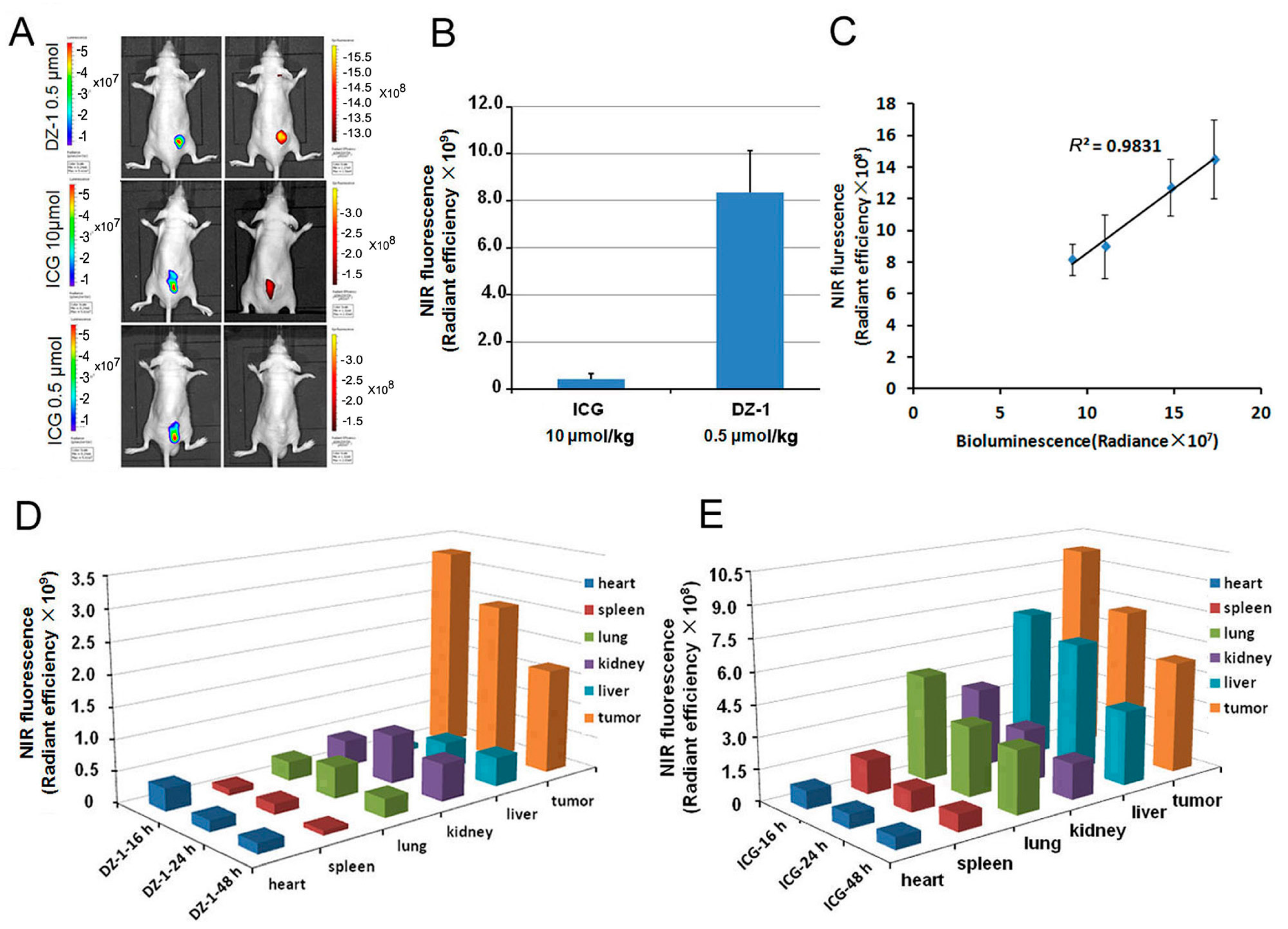
2.1. Uptake of ICG and DZ-1 by HCC
2.2. Organ Distribution of ICG and DZ-1 in Nude Mouse Model of HCC
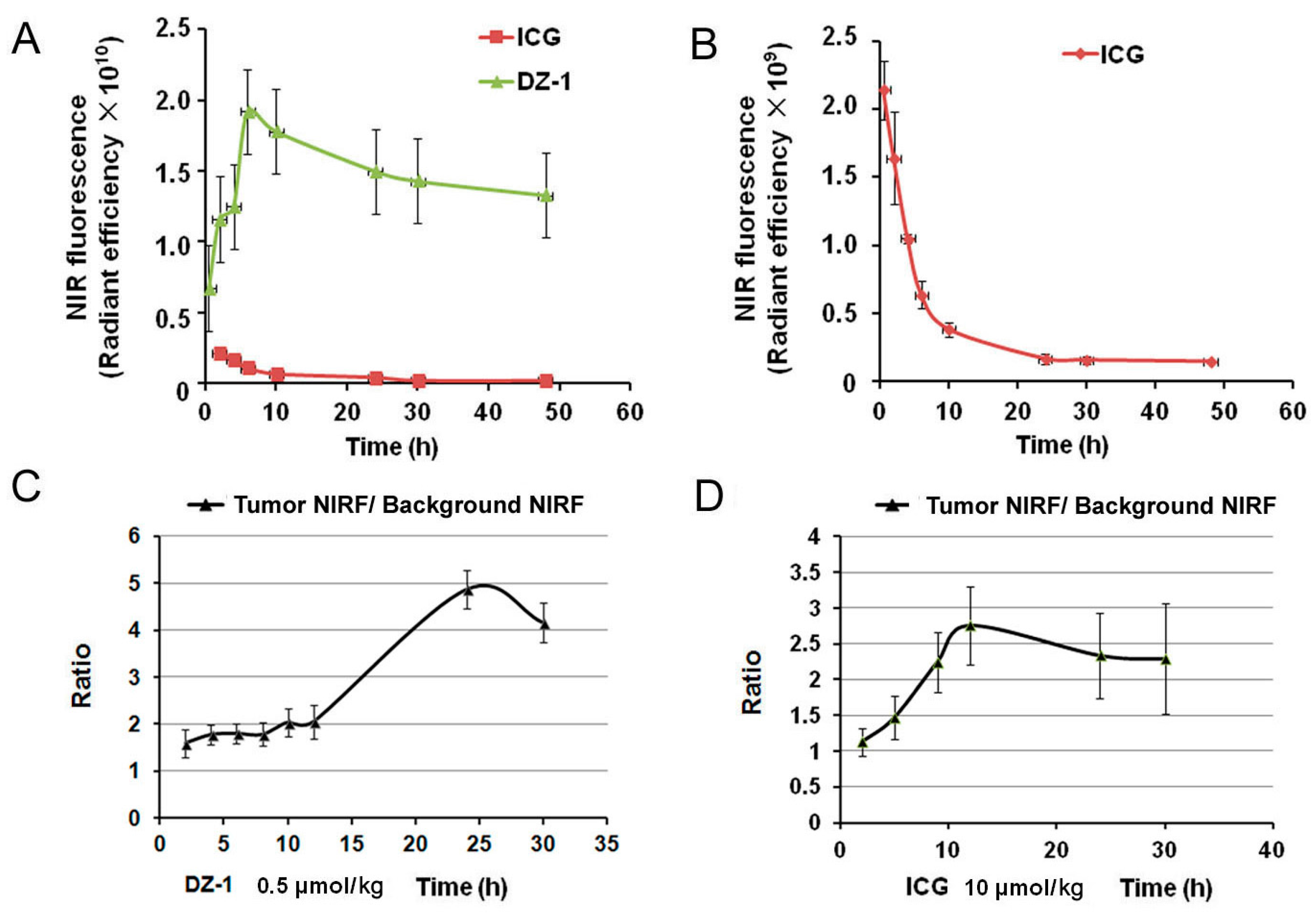
2.3. Metabolic Characteristics of ICG/DZ-1 in Nude Mice with Subcutaneous HCC Xenograft
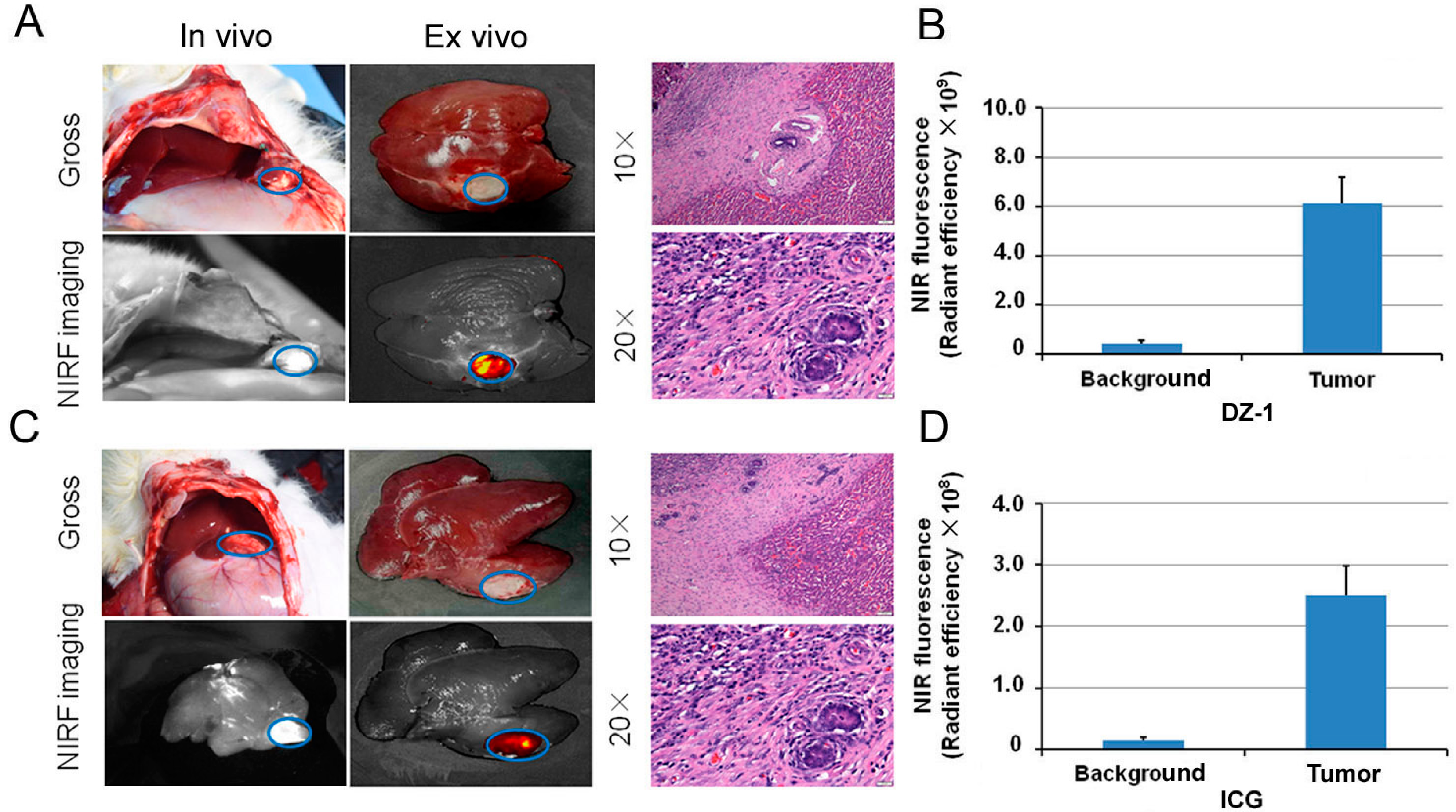
2.4. Imaging of ICG/DZ-1 in Nude Mice with Orthotopic HCC Xenograft
2.5. Intraoperative Navigation of Rabbit Liver Cancer Model by ICG/DZ-1
2.6. DZ-1-Mediated Targeted Delivery of Antitumor Drugs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Clinical Specimens
4.3. Reagents
4.4. The Emission Spectrum of DZ-1
4.5. Bioluminescence and NIRF Imaging for Tumor Xenograft Models
4.6. DZ-1 and ICG Uptake by Human HCC Cells
4.7. Metabolism of NIRF Dyes in Nude Mouse Subcutaneous Xenograft HCC Model
4.8. NIRF Dye Uptake by Orthotopic Liver Xenograft Model in Nude Mice
4.9. Imaging of NIRF Dye in Rabbit Liver Tumor Model
4.10. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NIRF | Near infrared fluorescence |
| ICG | Indocyanine green |
| HCC | Hepatocellular carcinoma |
| PDX | Patient-derived xenograft |
| F/T | Fluorescence intensity/tumor area |
| NIRG | Analog-gemcitabine conjugate |
| FMMU | Fourth Military Medical University |
| BLI | Bioluminescence |
References
- Reinier, H.; Sun, H.; England, C.G.; Valdovinos, H.F.; Ehlerding, E.B.; Barnhart, T.E.; Yang, Y.; Cai, W. CD146-targeted immunoPET and NIRF imaging of hepatocellular carcinoma with a dual-labeled monoclonal antibody. Theranostics 2016, 6, 1918–1933. [Google Scholar]
- Yang, G.; Yi, Z.; Li, X.; Jie, L.; Wang, J.P.; Chordia, M.D.; Zhong, Q.L.; Chung, L.W.K.; Wei, Y.; Pan, D. Improving therapeutic potential of farnesylthiosalicylic acid: Tumor specific delivery via conjugation with heptamethine cyanine dye. Mol. Pharm. 2016, 14, 1–13. [Google Scholar]
- Trivedi, E.R.; Harney, A.S.; Olive, M.B.; Podgorski, I.; Moin, K.; Sloane, B.F.; Barrett, A.G.; Meade, T.J.; Hoffman, B.M. Chiral porphyrazine near-IR optical imaging agent exhibiting preferential tumor accumulation. Proc. Natl. Acad. Sci. USA 2010, 107, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wu, J.B.; Pan, D. Review on near-infrared heptamethine cyanine dyes as theranostic agents for tumor imaging, targeting, and photodynamic therapy. J. Biomed. Opt. 2016, 21, 50901. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, F.; Qin, W.; Yang, X.; Yuan, J. Near-infrared fluorescent probes in cancer imaging and therapy: An emerging field. Int. J. Nanomed. 2014, 9, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. New technologies for human cancer imaging. J. Clin. Oncol. 2008, 26, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Chao, Y.K.; Liu, Y.H.; Wen, C.T.; Chen, W.H.; Wu, C.Y.; Hsieh, M.J.; Wu, Y.C.; Liu, H.P. Clinical use of near-infrared fluorescence imaging with indocyanine green in thoracic surgery: A literature review. J. Thorac. Dis. 2016, 8, S744–S748. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.S.; Ren, S.; Sun, X.; Chong, H.S. Theranostic polyaminocarboxylate-cyanine-transferrin conjugate for anti-cancer therapy and near IR optical imaging. ChemMedChem 2016, 11, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Faizi, M.S.H.; Rather, J.A.; Khan, M.S. Next generation NIR fluorophores for tumor imaging and fluorescence-guided surgery: A review. Bioorg. Med. Chem. 2017, 25, 2017–2034. [Google Scholar] [CrossRef] [PubMed]
- Liberale, G.; Vankerckhove, S.; Galdon, M.G.; Donckier, V.; Larsimont, D.; Bourgeois, P. Fluorescence imaging after intraoperative intravenous injection of indocyanine green for detection of lymph node metastases in colorectal cancer. Eur. J. Surg. Oncol. 2015, 41, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, C.; Tong, R.; Qian, W.; Zhau, H.E.; Wang, R.; Zhu, G.; Cheng, J.; Yang, V.W.; Cheng, T. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin. Cancer Res. 2010, 16, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Shi, C.; Chu, G.C.; Xu, Q.; Zhang, Y.; Li, Q.; Yu, J.S.; Zhau, H.E.; Chung, L.W. Near-infrared fluorescence heptamethine carbocyanine dyes mediate imaging and targeted drug delivery for human brain tumor. Biomaterials 2015, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Lin, T.P.; Gallagher, J.D.; Kushal, S.; Chung, L.W.; Zhau, H.E.; Olenyuk, B.Z.; Shih, J.C. Monoamine oxidase A inhibitor-near-infrared dye conjugate reduces prostate tumor growth. J. Am. Chem. Soc. 2015, 137, 2366–2374. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shao, C.; Wang, R.; Chu, C.Y.; Hu, P.; Master, V.; Osunkoya, A.O.; Kim, H.L.; Zhau, H.E.; Chung, L.W.K. Optical imaging of kidney cancer with novel near-infrared heptamethine carbocyanine fluorescent dyes. J. Urol. 2013, 189, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Kushal, S.; Wang, W.; Vaikari, V.P.; Kota, R.; Chen, K.; Yeh, T.S.; Jhaveri, N.; Groshen, S.L.; Olenyuk, B.Z.; Chen, T.C. Monoamine oxidase A (MAO A) inhibitors decrease glioma progression. Oncotarget 2016, 7, 13842–13853. [Google Scholar] [PubMed]
- Wu, J.; Pan, D.; Chung, L.W. Near-infrared fluorescence and nuclear imaging and targeting of prostate cancer. Transl. Androl. Urol. 2013, 2, 254–264. [Google Scholar] [PubMed]
- Shi, C.; Wu, J.B.; Chu, C.Y.; Li, Q.; Wang, R.; Zhang, C.; Zhang, Y.; Kim, H.L.; Wang, J.; Zhau, H.E. Heptamethine carbocyanine dye-mediated near-infrared imaging of canine and human cancers through the HIF-1α/OATPs signaling axis. Oncotarget 2014, 5, 10114–10126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, C.; Yong, Z.; Bing, B.; An, J.; Hai, Z.; Wu, J.B.; Shi, C. Optical imaging of gastric cancer with near-infrared heptamethine carbocyanine fluorescence dyes. Oncotarget 2016, 7, 57277–57289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, N.; Cao, Q.; Cui, X.; Zhou, Q.; Yang, C. The effects of propofol on the growth behavior of hepatoma xenografts in Balb/c mice. Biomed. Pharmacother. 2017, 90, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Levesque, E.; Martin, E.; Dudau, D.; Lim, C.; Dhonneur, G.; Azoulay, D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth. Crit. Care Pain Med. 2016, 35, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lisotti, A.; Azzaroli, F.; Buonfiglioli, F.; Montagnani, M.; Cecinato, P.; Turco, L.; Calvanese, C.; Simoni, P.; Guardigli, M.; Arena, R. Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology 2014, 59, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Ashitate, Y.; Vooght, C.S.; Hutteman, M.; Oketokoun, R.; Choi, H.S.; Frangioni, J.V. Simultaneous Assessment of luminal integrity and vascular perfusion of the gastrointestinal tract using dual-channel near-infrared fluorescence. Mol. Imaging 2012, 11, 301–308. [Google Scholar] [PubMed]
- Koyanagi, K.; Ozawa, S.; Oguma, J.; Kazuno, A.; Yamazaki, Y.; Ninomiya, Y.; Ochiai, H.; Tachimori, Y. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: New predictive evaluation of anastomotic leakage after esophagectomy. Medicine 2016, 95, e4386. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, T.; Oue, T.; Yonekura, T.; Owari, M.; Nakahata, K.; Umeda, S.; Nara, K.; Ueno, T.; Uehara, S.; Usui, N. Clinical application of indocyanine green (ICG) fluorescent imaging of hepatoblastoma. J. Pediatr. Surg. 2015, 50, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Juhl, K.; Christensen, A.; Persson, M.; Ploug, M.; Kjaer, A. Peptide-based optical uPAR imaging for surgery: In vivo testing of ICG-Glu-Glu-AE105. PLoS ONE 2016, 11, e0147428. [Google Scholar] [CrossRef] [PubMed]
- Keereweer, S.; Mol, I.M.; Kerrebijn, J.D.; van Driel, P.B.; Xie, B.; Rj, B.D.J.; Vahrmeijer, A.L.; Löwik, C.W. Targeting integrins and enhanced permeability and retention (EPR) effect for optical imaging of oral cancer. Oral Oncol. 2011, 47, S70–S71. [Google Scholar] [CrossRef]
- Kaibori, M.; Matsui, K.; Ishizaki, M.; Iida, H.; Sakaguchi, T.; Tsuda, T.; Okumura, T.; Inoue, K.; Shimada, S.; Ohtsubo, S. Evaluation of fluorescence imaging with indocyanine green in hepatocellular carcinoma. Cancer Imaging 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Von Driel, P.B.A.A.; Boonstra, M.C.; Prevoo, H.A.J.M.; van de Giessen, M.; Snoeks, T.J.A.; Tummers, Q.R.J.G.; Keereweer, S.; Cordfunke, R.A.; Fish, A.; van Eendenburg, J.D.H.; et al. EpCAM as multi-tumour target for near-infrared fluorescence guided surgery. BMC Cancer 2016, 16, 884. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.F.; Schaberle, F.A.; Monteiro, C.J.; Dąbrowski, J.M.; Arnaut, L.G. The challenging combination of intense fluorescence and high singlet oxygen quantum yield in photostable chlorins—A contribution to theranostics. Photochem. Photobiol. Sci. 2013, 12, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Shao, C.; Li, X.; Shi, C.; Li, Q.; Hu, P.; Chen, Y.T.; Dou, X.; Sahu, D.; Li, W. Near-infrared fluorescence imaging of cancer mediated by tumor hypoxia and HIF1α/OATPs signaling axis. Biomaterials 2014, 35, 8175–8185. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, K.; Thakkar, N.; Kim, D.; Lee, W. Role of hypoxia inducible factor-1α in the regulation of the cancer-specific variant of organic anion transporting polypeptide 1B3 (OATP1B3), in colon and pancreatic cancer. Biochem. Pharmacol. 2013, 86, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fan, C.; Livasy, C.; He, X.; Oh, D.S.; Ewend, M.G.; Carey, L.A.; Surbramanian, S.; West, R.; Ikpatt, F.; et al. A compact VEGF signature associated with distant metastases and poor outcomes. BMC Med. 2009, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Luo, S.; Tan, X.; Shi, C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials 2014, 35, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Stackow, R.; Trevithick-Sutton, C.; Foote, C.S.; Barrett, A.G.; Hoffman, B.M. Tuning the singlet oxygen quantum yield of near-IR-absorbing porphyrazines. Photochem. Photobiol. 2003, 77, 18–21. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Sakaguchi, T.; Hiraide, T.; Morita, Y.; Suzuki, A.; Baba, S.; Setou, M.; Konno, H. Expression of indocyanine green-related transporters in hepatocellular carcinoma. J. Surg. Res. 2014, 193, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, T.; Masuda, K.; Urano, Y.; Kawaguchi, Y.; Satou, S.; Kaneko, J.; Hasegawa, K.; Shibahara, J.; Fukayama, M.; Tsuji, S. Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Onda, N.; Kimura, M.; Yoshida, T.; Shibutani, M. Preferential tumor cellular uptake and retention of indocyanine green for in vivo tumor imaging. Int. J. Cancer 2016, 139, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. Off. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; Possinger, K.; Phillip, P.; Beykirch, M.; Kerr, H.; Walling, J.; Harris, A.L. Advanced breast cancer: A phase II trial with gemcitabine. J. Clin. Oncol. Off. 1995, 13, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Exp. Rev. Mol. Med. 2011, 13, e17. [Google Scholar] [CrossRef] [PubMed]
- Lawson, H.C.; Bohan, P.S.E.; Park, M.C.; Hussain, N.; Olivi, A.; Weingart, J.; Kleinberg, L.; Brem, H. Interstitial chemotherapy for malignant gliomas: The Johns Hopkins experience. J. Neuro Oncol. 2007, 83, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Du, Y.; Ye, J.; Kou, D.; Qiu, J.; Wang, J.; Tian, J.; Chen, X. Intraoperative imaging-guided cancer surgery: From current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics 2014, 4, 1072–1084. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhao, Y.; Zhang, H.; Chen, X.; Zhao, N.; Tan, D.; Zhang, H.; Shi, C. The Application of Heptamethine Cyanine Dye DZ-1 and Indocyanine Green for Imaging and Targeting in Xenograft Models of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2017, 18, 1332. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061332
Zhang C, Zhao Y, Zhang H, Chen X, Zhao N, Tan D, Zhang H, Shi C. The Application of Heptamethine Cyanine Dye DZ-1 and Indocyanine Green for Imaging and Targeting in Xenograft Models of Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2017; 18(6):1332. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061332
Chicago/Turabian StyleZhang, Caiqin, Yong Zhao, He Zhang, Xue Chen, Ningning Zhao, Dengxu Tan, Hai Zhang, and Changhong Shi. 2017. "The Application of Heptamethine Cyanine Dye DZ-1 and Indocyanine Green for Imaging and Targeting in Xenograft Models of Hepatocellular Carcinoma" International Journal of Molecular Sciences 18, no. 6: 1332. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061332




