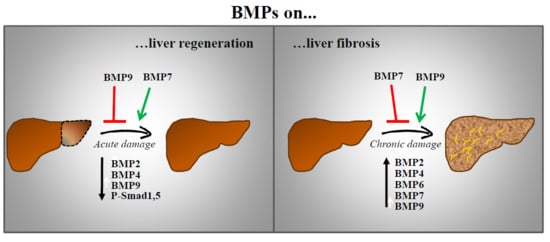
BMP Signalling at the Crossroad of Liver Fibrosis and Regeneration
Abstract
:1. Introduction to BMPs
2. BMP Signalling: Canonical and Non-Canonical Pathways
2.1. BMP Receptors
2.2. Smad-Dependent Pathway
2.3. Non-Smad Pathways
2.4. BMP Signalling Regulators
3. BMP and Liver Physiology
4. BMP and Liver Regeneration
4.1. BMPs in Hepatocyte-Mediated Liver Regeneration
4.2. BMPS in Liver Regeneration during Chronic Liver Injury
5. BMPs and Liver Fibrosis
Perspective on BMP Signalling as Therapeutic Target in Liver Fibrosis
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BMP | Bone Morphogenetic Proteins |
| GDF | Growth Differentiation Factor |
| SBE | Smad-binding elements |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphatidylinositol 3-kinase |
| RGM | Repulsive guidance molecules |
| RTK | Receptor tyrosine kinases |
| PH | Partial hepatectomy |
| LPC | Liver progenitor cell |
| DDC | 3,5-Diethoxycarbonyl-1,4-dihydrocollidine |
| EMT | Epithelial to mesenchymal transition |
| HSC | Hepatic stellate cells |
| αSMA | α-Smooth muscle actin |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NASH | Non-alcoholic steatohepatitis |
| KPC | Kielin/chordin-like protein |
| CTGF | Connective tissue growth factor |
| USAG1 | Uterine sensitization-associated gene-1 |
| TLR4 | Toll like Receptor 4 |
References
- Rider, C.; Mulloy, B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem. J. 2010, 429, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Bezooijen, R.L.; Heldin, C.-H.; ten Dijke, P. Bone morphogenetic proteins and their receptors. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Yadin, D.; Knaus, P.; Mueller, T.D. Structural insights into BMP receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 2016, 27, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Little, S.C.; Mullins, M.C. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat. Cell Biol. 2009, 11, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Alves, G.G.; Zambuzzi, W.F.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Structure, biological function and therapeutic applications. Arch. Biochem. Biophys. 2014, 561, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Duffhues, G.; Hiepen, C.; Knaus, P.; Ten Dijke, P. Bone morphogenetic protein signalling in bone homeostasis. Bone 2015, 80, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L. Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev. 1996, 6, 432–438. [Google Scholar] [CrossRef]
- Wagner, D.O.; Sieber, C.; Bhushan, R.; Borgermann, J.H.; Graf, D.; Knaus, P. BMPs: From bone to body morphogenetic proteins. Sci. Signal. 2010, 3, mr1. [Google Scholar] [PubMed]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone morphogenetic proteins: A critical review. Cell. Signal. 2011, 23, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Moustakas, A. Signalling receptors for TGF-β family members. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signalling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP signalling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.; Kopf, J.; Hiepen, C.; Knaus, P. Recent advances in BMP receptor signalling. Cytokine Growth Factor Rev. 2009, 20, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-Smad pathways in TGF-β signalling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Conidi, A.; Cazzola, S.; Beets, K.; Coddens, K.; Collart, C.; Cornelis, F.; Cox, L.; Joke, D.; Dobreva, M.P.; Dries, R.; et al. Few Smad proteins and many Smad-interacting proteins yield multiple functions and action modes in TGFβ/BMP signalling in vivo. Cytokine Growth Factor Rev. 2011, 22, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Miyazono, K. Regulation of TGF-β family signalling by inhibitory Smads. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Horbelt, D.; Denkis, A.; Knaus, P. A portrait of Transforming Growth Factor beta superfamily signalling: Background matters. Int. J. Biochem. Cell Biol. 2012, 44, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.E. TGFβ signalling in the development of ovarian function. Cell Tissue Res. 2005, 322, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Holtzhausen, A.; Golzio, C.; How, T.; Lee, Y.H.; Schiemann, W.P.; Katsanis, N.; Blobe, G.C. Novel bone morphogenetic protein signaling through Smad2 and Smad3 to regulate cancer progression and development. FASEB J. 2014, 28, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.P.; Yeo, C.Y.; Lee, Y.; Schrewe, H.; Whitman, M.; Li, E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002, 16, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Cheng, F.; Du, X.T.; Gao, J.L.; Xiao, X.L.; Li, N.; Li, S.L.; Dong, D.L. Correction: GDF11/BMP11 activates both smad1/5/8 and smad2/3 signals but shows no significant effect on proliferation and migration of human umbilical vein endothelial cells. Oncotarget 2016, 7, 46832. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.H. The regulation of TGFβ signal transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Hill, C.S. How the Smads regulate transcription. Int. J. Biochem. Cell Biol. 2008, 40, 383–408. [Google Scholar] [CrossRef] [PubMed]
- Wotton, D.; Massague, J. Smad transcriptional corepressors in TGF β family signaling. Curr. Top. Microbiol. Immunol. 2001, 254, 145–164. [Google Scholar] [PubMed]
- Herpin, A.; Cunningham, C. Cross-talk between the bone morphogenetic protein pathway and other major signaling pathways results in tightly regulated cell-specific outcomes. FEBS J. 2007, 274, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Leung-Hagesteijn, C.; Hu, M.C.; Mahendra, A.S.; Hartwig, S.; Klamut, H.J.; Rosenblum, N.D.; Hannigan, G.E. Integrin-linked kinase mediates bone morphogenetic protein 7-dependent renal epithelial cell morphogenesis. Mol. Cell. Biol. 2005, 25, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Choudhury, N.; Abboud, S.L.; Nishimura, R.; Celeste, A.; Mahimainathan, L.; Choudhury, G.G. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J. Biol. Chem. 2002, 277, 33361–33368. [Google Scholar] [CrossRef] [PubMed]
- Guzman, A.; Zelman-Femiak, M.; Boergermann, J.H.; Paschkowsky, S.; Kreuzaler, P.A.; Fratzl, P.; Harms, G.S.; Knaus, P. SMAD versus non-SMAD signaling is determined by lateral mobility of bone morphogenetic protein (BMP) receptors. J. Biol. Chem. 2012, 287, 39492–39504. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Kang, H. Functions of the bone morphogenetic protein signaling pathway through microRNAs. Int. J. Mol. Med. 2015, 35, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Zakin, L.; De Robertis, E.M. Extracellular regulation of BMP signaling. Curr. Biol. 2010, 20, R89–R92. [Google Scholar] [CrossRef] [PubMed]
- Heinke, J.; Wehofsits, L.; Zhou, Q.; Zoeller, C.; Baar, K.M.; Helbing, T.; Laib, A.; Augustin, H.; Bode, C.; Patterson, C.; et al. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ. Res. 2008, 103, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Kattamuri, C.; Ozdeslik, R.N.; Schmiedel, C.; Mentzer, S.; Schorl, C.; Oancea, E.; Thompson, T.B.; Fallon, J.R. MuSK is a BMP co-receptor that shapes BMP responses and calcium signaling in muscle cells. Sci. Signal. 2016, 9, ra87. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Yun, C.; Kim, H.S.; Kim, S.J. TrkC binds to the bone morphogenetic protein type II receptor to suppress bone morphogenetic protein signaling. Cancer Res. 2007, 67, 9869–9877. [Google Scholar] [CrossRef] [PubMed]
- Sammar, M.; Stricker, S.; Schwabe, G.C.; Sieber, C.; Hartung, A.; Hanke, M.; Oishi, I.; Pohl, J.; Minami, Y.; Sebald, W.; et al. Modulation of GDF5/BRI-b signalling through interaction with the tyrosine kinase receptor Ror2. Genes Cells 2004, 9, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Onichtchouk, D.; Chen, Y.G.; Dosch, R.; Gawantka, V.; Delius, H.; Massague, J.; Niehrs, C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 1999, 401, 480–485. [Google Scholar] [PubMed]
- Luo, J.Y.; Zhang, Y.; Wang, L.; Huang, Y. Regulators and effectors of bone morphogenetic protein signalling in the cardiovascular system. J. Physiol. 2015, 593, 2995–3011. [Google Scholar] [CrossRef] [PubMed]
- Tsubakihara, Y.; Hikita, A.; Yamamoto, S.; Matsushita, S.; Matsushita, N.; Oshima, Y.; Miyazawa, K.; Imamura, T. Arkadia enhances BMP signalling through ubiquitylation and degradation of Smad6. J. Biochem. 2015, 158, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Herhaus, L.; Sapkota, G.P. The emerging roles of deubiquitylating enzymes (DUBs) in the TGFβ and BMP pathways. Cell. Signal 2014, 26, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xie, F.; Jin, K.; Zhang, Z.; Clerici, M.; Gao, R.; van Dinther, M.; Sixma, T.K.; Huang, H.; Zhang, L.; et al. USP4 inhibits SMAD4 monoubiquitination and promotes activin and BMP signaling. EMBO J. 2017, 36, 1623–1639. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; King, D.; Young, K.; Litchfield, D.W.; Petersen, N.O.; Nohe, A. Casein kinase 2 β-subunit is a regulator of bone morphogenetic protein 2 signaling. Biophys. J. 2010, 99, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Haney, J.C.; Sogani, J.; Blobe, G.C. Casein kinase 2β as a novel enhancer of activin-like receptor-1 signaling. FASEB J. 2009, 23, 3712–3721. [Google Scholar] [CrossRef] [PubMed]
- Dorpholz, G.; Murgai, A.; Jatzlau, J.; Horbelt, D.; Belverdi, M.P.; Heroven, C.; Schreiber, I.; Wendel, G.; Ruschke, K.; Stricker, S.; et al. IRS4, a novel modulator of BMP/Smad and Akt signalling during early muscle differentiation. Sci. Rep. 2017, 7, 8778. [Google Scholar] [CrossRef] [PubMed]
- Itasaki, N.; Hoppler, S. Crosstalk between Wnt and bone morphogenic protein signaling: A turbulent relationship. Dev. Dyn. 2010, 239, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Munnamalai, V.; Fekete, D.M. Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner. Development 2016, 143, 4003–4015. [Google Scholar] [CrossRef] [PubMed]
- Finelli, M.J.; Murphy, K.J.; Chen, L.; Zou, H. Differential phosphorylation of Smad1 integrates BMP and neurotrophin pathways through Erk/Dusp in axon development. Cell Rep. 2013, 3, 1592–1606. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, G.; Alarcon, C.; Spagnoli, F.M.; Brivanlou, A.H.; Massague, J. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol. Cell 2007, 25, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Aykul, S.; Martinez-Hackert, E. Transforming growth factor-β family ligands can function as antagonists by competing for type II receptor binding. J. Biol. Chem. 2016, 291, 10792–10804. [Google Scholar] [CrossRef] [PubMed]
- Core, A.B.; Canali, S.; Babitt, J.L. Hemojuvelin and bone morphogenetic protein (BMP) signaling in iron homeostasis. Front. Pharmacol. 2014, 5, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grgurevic, L.; Christensen, G.L.; Schulz, T.J.; Vukicevic, S. Bone morphogenetic proteins in inflammation, glucose homeostasis and adipose tissue energy metabolism. Cytokine Growth Factor Rev. 2016, 27, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Lellis-Santos, C.; Leite, A.R.; Hirabara, S.M.; Boschero, A.C.; Curi, R.; Anhe, G.F.; Bordin, S. Smad5 regulates Akt2 expression and insulin-induced glucose uptake in L6 myotubes. Mol. Cell. Endocrinol. 2010, 319, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Grzegorzewski, K.J.; Barash, S.; Zhao, Q.; Schneider, H.; Wang, Q.; Singh, M.; Pukac, L.; Bell, A.C.; Duan, R.; et al. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat. Biotechnol. 2003, 21, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, L.; Xu, X.; Wu, T.; Yang, M.; Zhang, C.; Mou, H.; Zhou, T.; Jia, Y.; Cai, C.; et al. Decreased circulating BMP-9 levels in patients with Type 2 diabetes is a signature of insulin resistance. Clin. Sci. 2017, 131, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Bruun, C.; Christensen, G.L.; Jacobsen, M.L.; Kanstrup, M.B.; Jensen, P.R.; Fjordvang, H.; Mandrup-Poulsen, T.; Billestrup, N. Inhibition of beta cell growth and function by bone morphogenetic proteins. Diabetologia 2014, 57, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Goulley, J.; Dahl, U.; Baeza, N.; Mishina, Y.; Edlund, H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007, 5, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Engberding, N.; Dikalova, A.E.; Chang, K.H.; Seidel-Rogol, B.; Long, J.S.; Lassegue, B.; Jo, H.; Griendling, K.K. The bone morphogenic protein inhibitor, noggin, reduces glycemia and vascular inflammation in db/db mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H747–H755. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.W.; Tang, Y.; Li, X.; Liu, Y.; Zhang, Y.Y.; Huang, H.Y.; Xue, R.D.; Yu, H.Y.; Guo, L.; Gao, H.D.; et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, E798–E807. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.J.; Tseng, Y.H. Brown adipose tissue: Development, metabolism and beyond. Biochem. J. 2013, 453, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Thorgeirsson, S.S. Hepatic stem cells in liver regeneration. FASEB J. 1996, 10, 1249–1256. [Google Scholar] [PubMed]
- Knight, B.; Matthews, V.B.; Olynyk, J.K.; Yeoh, G.C. Jekyll and Hyde: Evolving perspectives on the function and potential of the adult liver progenitor (oval) cell. Bioessays 2005, 27, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.A.; Glorioso, J.M.; Nyberg, S.L. Liver regeneration. Transl. Res. 2014, 163, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Otu, H.H.; Naxerova, K.; Ho, K.; Can, H.; Nesbitt, N.; Libermann, T.A.; Karp, S.J. Restoration of liver mass after injury requires proliferative and not embryonic transcriptional patterns. J. Biol. Chem. 2007, 282, 11197–11204. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.P.; Ji, W.M.; van den Brink, G.R.; Peppelenbosch, M.P. Bone morphogenetic protein-2 is a negative regulator of hepatocyte proliferation downregulated in the regenerating liver. World J. Gastroenterol. 2006, 12, 7621–7625. [Google Scholar] [CrossRef] [PubMed]
- Do, N.; Zhao, R.; Ray, K.; Ho, K.; Dib, M.; Ren, X.; Kuzontkoski, P.; Terwilliger, E.; Karp, S.J. BMP4 is a novel paracrine inhibitor of liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1220–G1227. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, D.; Oya, Y.; Masuzaki, R.; Ray, K.; Engers, D.W.; Dib, M.; Do, N.; Kuramitsu, K.; Ho, K.; Frist, A.; et al. Specific activin receptor-like kinase 3 inhibitors enhance liver regeneration. J. Pharmacol. Exp. Ther. 2014, 351, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Breitkopf-Heinlein, K.; Meyer, C.; Konig, C.; Gaitantzi, H.; Addante, A.; Thomas, M.; Wiercinska, E.; Cai, C.; Li, Q.; Wan, F.; et al. BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut 2017, 66, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Yang, C.; LeBleu, V.S.; Soubasakos, M.A.; Giraldo, M.; Zeisberg, M.; Kalluri, R. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J. 2007, 21, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kan, N.G.; Junghans, D.; Izpisua Belmonte, J.C. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. FASEB J. 2009, 23, 3516–3525. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Luque, J.; Caballero-Diaz, D.; Martinez-Palacian, A.; Roncero, C.; Moreno-Caceres, J.; Garcia-Bravo, M.; Grueso, E.; Fernandez, A.; Crosas-Molist, E.; Garcia-Alvaro, M.; et al. Dissecting the role of epidermal growth factor receptor catalytic activity during liver regeneration and hepatocarcinogenesis. Hepatology 2016, 63, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, S.; Bowen, W.C.; Mars, W.M.; Orr, A.; Haynes, M.M.; DeFrances, M.C.; Liu, S.; Tseng, G.C.; Tsagianni, A.; Michalopoulos, G.K. Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation. Hepatology 2016, 64, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Huang, Y.L.; Yang, W.H.; Tang, C.H. Hepatocyte growth factor-induced BMP-2 expression is mediated by c-Met receptor, FAK, JNK, Runx2 and p300 pathways in human osteoblasts. Int. Immunopharmacol. 2012, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, S.; Kitano, S.; Karasaki, M.; Tsunemi, S.; Sano, H.; Iwasaki, T. Blocking c-Met signaling enhances bone morphogenetic protein-2-induced osteoblast differentiation. FEBS Open Bio 2015, 5, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Terai, H.; Nomura-Furuwatari, C.; Mizuno, S.; Matsumoto, K.; Nakamura, T.; Takaoka, K. Hepatocyte growth factor contributes to fracture repair by upregulating the expression of BMP receptors. J. Bone Miner. Res. 2005, 20, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Renzi, A.; Franchitto, A.; Cardinale, V.; Onori, P.; Reid, L.; Alvaro, D.; Gaudio, E. Stem/progenitor cell niches involved in hepatic and biliary regeneration. Stem Cells Int. 2016, 2016, 3658013. [Google Scholar] [CrossRef] [PubMed]
- Kohn-Gaone, J.; Gogoi-Tiwari, J.; Ramm, G.A.; Olynyk, J.K.; Tirnitz-Parker, J.E. The role of liver progenitor cells during liver regeneration, fibrogenesis and carcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G143–G154. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T. Stem/progenitor cells in liver regeneration. Hepatology 2016, 64, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Miyajima, A. Liver regeneration by stem/progenitor cells. Hepatology 2014, 59, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Shen, H.; Dai, Q.; Minuk, G.Y.; Burzynski, F.J.; Gong, Y. Bone morphogenetic protein-4 induced rat hepatic progenitor cell (WB-F344 cell) differentiation toward hepatocyte lineage. J. Cell. Physiol. 2009, 220, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Yuan, X.H.; Shen, H. BMP-4 induced proliferation and oriented differentiation of rat hepatic oval cells into hepatocytes. Asian Pac. J. Trop. Med. 2015, 8, 412–416. [Google Scholar] [CrossRef]
- Goto, F.; Kakinuma, S.; Miyoshi, M.; Tsunoda, T.; Kaneko, S.; Sato, A.; Asano, Y.; Otani, S.; Azuma, S.; Nagata, H.; et al. Bone morphogenetic protein-4 modulates proliferation and terminal differentiation of fetal hepatic stem/progenitor cells. Hepatol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gouon-Evans, V.; Boussemart, L.; Gadue, P.; Nierhoff, D.; Koehler, C.I.; Kubo, A.; Shafritz, D.A.; Keller, G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat. Biotechnol. 2006, 24, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Raya, A.; Kawakami, Y.; Morita, M.; Matsui, T.; Nakashima, K.; Gage, F.H.; Rodriguez-Esteban, C.; Izpisua Belmonte, J.C. Nanog binds to Smad1 and blocks bone morphogenetic protein-induced differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10294–10299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, H.; Zhao, F.; Lu, P.; Ge, C.; Li, H.; Hou, H.; Yan, M.; Chen, T.; Jiang, G.; et al. BMP4 administration induces differentiation of CD133+ hepatic cancer stem cells, blocking their contributions to hepatocellular carcinoma. Cancer Res. 2012, 72, 4276–4285. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhao, Y.; Liu, Y.; Ye, F.; Song, Z.; Qin, H.; Meng, S.; Chen, Y.; Zhou, R.; Song, X.; et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology 2007, 45, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Si-Tayeb, K.; Noto, F.K.; Nagaoka, M.; Li, J.; Battle, M.A.; Duris, C.; North, P.E.; Dalton, S.; Duncan, S.A. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 2010, 51, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, R.; Taniguchi, M.; Hirata, M.; Shiota, G.; Sato, K. Transient expression of bone morphogenic protein-2 in acute liver injury by carbon tetrachloride. J. Biochem. 2007, 141, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.Y.; Khaliq, M.; Tsurusaki, S.; Ninov, N.; Stainier, D.Y.R.; Tanaka, M.; Shin, D. Bmp signaling governs biliary-driven liver regeneration in zebrafish via Tbx2b and Id2a. Hepatology 2017, 66, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Spath, G.F.; Weiss, M.C. Hepatocyte nuclear factor 4 provokes expression of epithelial marker genes, acting as a morphogen in dedifferentiated hepatoma cells. J. Cell Biol. 1998, 140, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ning, G.; Duncan, S.A. Mammalian hepatocyte differentiation requires the transcription factor HNF-4α. Genes Dev. 2000, 14, 464–474. [Google Scholar] [PubMed]
- Addante, A.R.C.; Almalé, L.; Lazcanoiturburu, N.; García-Álvaro, M.; Fernández, M.; Sanz, J.; Lee, S.-J.; Fabregat, I.; Dooley, S.; ten Dijke, P.; et al. Novel role of Bone Morphogenetic Protein 9 as regulator of liver progenitor cells in a mouse model of liver cholestasis. (unpublished, manuscript in preparation).
- Novo, E.; Cannito, S.; Morello, E.; Paternostro, C.; Bocca, C.; Miglietta, A.; Parola, M. Hepatic myofibroblasts and fibrogenic progression of chronic liver diseases. Histol. Histopathol. 2015, 30, 1011–1032. [Google Scholar] [PubMed]
- Liu, Y.; Meyer, C.; Xu, C.; Weng, H.; Hellerbrand, C.; ten Dijke, P.; Dooley, S. Animal models of chronic liver diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G449–G468. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C. Current and future anti-fibrotic therapies for chronic liver disease. Clin. Liver Dis. 2008, 12, 939–962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.C.; Zhang, Q.B.; Qiao, L. Pathogenesis of liver cirrhosis. World J. Gastroenterol. 2014, 20, 7312–7324. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Caceres, J.; Sanchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Ten Dijke, P.; Consortium, I.-L. TGF-β signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Meurer, S.K.; Gressner, O.A.; Herrmann, J.; Borkham-Kamphorst, E.; Gressner, A.M. BMP-7 as antagonist of organ fibrosis. Front. Biosci. 2009, 14, 4992–5012. [Google Scholar] [CrossRef]
- Li, R.X.; Yiu, W.H.; Tang, S.C. Role of bone morphogenetic protein-7 in renal fibrosis. Front. Physiol. 2015, 6, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.M.; Chung, A.C.; Lan, H.Y. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin. Sci. (Lond.) 2013, 124, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Kalluri, R. Reversal of experimental renal fibrosis by BMP7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatr. Nephrol. 2008, 23, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Iimuro, Y.; Otogawa, K.; Saika, S.; Inagaki, Y.; Nakajima, Y.; Kawada, N.; Fujimoto, J.; Friedman, S.L.; Ikeda, K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 2007, 56, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.M.; Cai, M.; Lv, Y.F.; Huang, Y.H.; Li, H.H. Oral administration of recombinant adeno-associated Virus-mediated bone morphogenetic protein-7 suppresses CCl4-induced hepatic fibrosis in mice. Mol. Ther. 2012, 20, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.N.; Tuleuova, N.; Lee, J.Y.; Ramanculov, E.; Reddi, A.H.; Zern, M.A.; Revzin, A. Cultivating hepatocytes on printed arrays of HGF and BMP7 to characterize protective effects of these growth factors during in vitro alcohol injury. Biomaterials 2010, 31, 5936–5944. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.M.; Su, H.L.; Li, C.; Lin, S.Z.; Yen, S.Y.; Huang, M.H.; Ho, L.I.; Chiou, T.W.; Harn, H.J. The Role of Butylidenephthalide in Targeting the Microenvironment Which Contributes to Liver Fibrosis Amelioration. Front. Pharmacol. 2016, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Liu, R.; Liu, X.; Cui, L.; Wen, Y.; Yan, S.; Yin, C. Attenuation of liver fibrosis by herbal compound 861 via upregulation of BMP-7/Smad signaling in the bile duct ligation model rat. Mol. Med. Rep. 2016, 13, 4335–4342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.K.; Cheng, M.L.; Wu, R.M.; Yao, Y.M.; Mu, M.; Zhu, J.J.; Zhang, B.F.; Zhou, M.Y. Effect of Danshao Huaxian capsule on Gremlin and bone morphogenetic protein-7 expression in hepatic fibrosis in rats. World J. Gastroenterol. 2014, 20, 14875–14883. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Peng, J.; Li, Q.F.; Yang, M.; Wang, Y.; Chen, W. Exogenous bone morphogenetic protein-7 reduces hepatic fibrosis in Schistosoma japonicum-infected mice via transforming growth factor-β/Smad signaling. World J. Gastroenterol. 2013, 19, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Dong, J.Z.; Xiong, L.J.; Shi, K.Q.; Zou, Z.L.; Zhang, S.N.; Cao, S.T.; Lin, Z.; Chen, Y.P. BMP-7 attenuates liver fibrosis via regulation of epidermal growth factor receptor. Int. J. Clin. Exp. Pathol. 2014, 7, 3537–3547. [Google Scholar] [PubMed]
- Wang, S.L.; Yang, C.Q.; Qi, X.L.; Yuan, M.; Chang, Y.Z.; Yang, L.; Gao, H.J. Inhibitory effect of bone morphogenetic protein-7 on hepatic fibrosis in rats. Int. J. Clin. Exp. Pathol. 2013, 6, 897–903. [Google Scholar] [PubMed]
- Zeisberg, M.; Hanai, J.; Sugimoto, H.; Mammoto, T.; Charytan, D.; Strutz, F.; Kalluri, R. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003, 9, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hirschberg, R. Bone morphogenetic protein-7 signals opposing transforming growth factor β in mesangial cells. J. Biol. Chem. 2004, 279, 23200–23206. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.D.; Phillips, A.; Fraser, D. Bone morphogenetic protein-7 inhibits proximal tubular epithelial cell Smad3 signaling via increased SnoN expression. Am. J. Pathol. 2010, 176, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Motazed, R.; Colville-Nash, P.; Kwan, J.T.; Dockrell, M.E. BMP-7 and proximal tubule epithelial cells: Activation of multiple signaling pathways reveals a novel anti-fibrotic mechanism. Pharm. Res. 2008, 25, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Izumi, N.; Mizuguchi, S.; Inagaki, Y.; Saika, S.; Kawada, N.; Nakajima, Y.; Inoue, K.; Suehiro, S.; Friedman, S.L.; Ikeda, K. BMP-7 opposes TGF-β1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L120–L126. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Gabele, E.; Bataille, F.; Schwabe, R.F.; Hellerbrand, C.; Klebl, F.; Straub, R.H.; Luedde, T.; Manns, M.P.; Trautwein, C.; et al. Bone morphogenetic protein 7 is elevated in patients with chronic liver disease and exerts fibrogenic effects on human hepatic stellate cells. Dig. Dis. Sci. 2007, 52, 3404–3415. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.R.; Xu, G.T.; Lv, L.X.; Yang, C.Q. The ratio of transforming growth factor-β1/bone morphogenetic protein-7 in the progression of the epithelial-mesenchymal transition contributes to rat liver fibrosis. Genet. Mol. Res. 2014, 13, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Shen, H.; Sun, Y.; Li, P.; Burczynski, F.; Namaka, M.; Gong, Y. Bone morphogenetic protein 4 mediates bile duct ligation induced liver fibrosis through activation of Smad1 and ERK1/2 in rat hepatic stellate cells. J. Cell. Physiol. 2006, 207, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Shen, W.F.; Ning, B.F.; Hu, P.F.; Lin, Y.; Yue, H.Y.; Yin, C.; Hou, J.L.; Chen, Y.X.; Zhang, J.P.; et al. Inhibition of extracellular signal-regulated kinase 1 by adenovirus mediated small interfering RNA attenuates hepatic fibrosis in rats. Hepatology 2009, 50, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Rowe, I.A.; Galsinh, S.K.; Wilson, G.K.; Parker, R.; Durant, S.; Lazar, C.; Branza-Nichita, N.; Bicknell, R.; Adams, D.H.; Balfe, P.; et al. Paracrine signals from liver sinusoidal endothelium regulate hepatitis C virus replication. Hepatology 2014, 59, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Hsia, Y.; Yang, W.Y.; Lin, Y.I.; Li, C.C.; Tsai, T.F.; Chang, K.W.; Shieh, G.S.; Tsai, S.F.; Wang, H.D.; et al. Identification of the common regulators for hepatocellular carcinoma induced by hepatitis B virus X antigen in a mouse model. Carcinogenesis 2012, 33, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Oumi, N.; Taniguchi, K.A.; Kanai, A.M.; Yasunaga, M.; Nakanishi, T.; Sato, K. A crucial role of bone morphogenetic protein signaling in the wound healing response in acute liver injury induced by carbon tetrachloride. Int. J. Hepatol. 2012, 2012, 476820. [Google Scholar] [CrossRef] [PubMed]
- Gerjevic, L.N.; Liu, N.; Lu, S.; Harrison-Findik, D.D. Alcohol activates TGF-Β but inhibits BMP receptor-mediated Smad signaling and Smad4 binding to hepcidin promoter in the liver. Int. J. Hepatol. 2012, 2012, 459278. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Huang, G.; Hadi, M.; Choy, P.; Zhang, M.; Minuk, G.Y.; Chen, Y.; Gong, Y. Transforming growth factor-β1 downregulation of Smad1 gene expression in rat hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G539–G546. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Wacker, E.; Dorn, C.; Koch, A.; Saugspier, M.; Thasler, W.E.; Hartmann, A.; Bosserhoff, A.K.; Hellerbrand, C. Enhanced expression of BMP6 inhibits hepatic fibrosis in non-alcoholic fatty liver disease. Gut 2015, 64, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gu, X.; Weng, H.; Ghafoory, S.; Liu, Y.; Feng, T.; Dzieran, J.; Li, L.; Ilkavets, I.; Kruithof-de Julio, M.; et al. Bone morphogenetic protein-9 (BMP-9) induces epithelial to mesenchymal transition (EMT) in hepatocellular carcinoma cells. Cancer Sci. 2013, 104, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Felix, J.M.; Cuesta, C.; Perretta-Tejedor, N.; Subileau, M.; Lopez-Hernandez, F.J.; Lopez-Novoa, J.M.; Martinez-Salgado, C. Identification of bone morphogenetic protein 9 (BMP9) as a novel profibrotic factor in vitro. Cell. Signal. 2016, 28, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Levet, S.; Ouarne, M.; Ciais, D.; Coutton, C.; Subileau, M.; Mallet, C.; Ricard, N.; Bidart, M.; Debillon, T.; Faravelli, F.; et al. BMP9 and BMP10 are necessary for proper closure of the ductus arteriosus. Proc. Natl. Acad. Sci. USA 2015, 112, E3207–E3215. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; Nunez, O.; Lorente, R.; Rincon, D.; Matilla, A.; Salcedo, M.; Catalina, M.V.; Ripoll, C.; Iacono, O.L.; Banares, R.; et al. Increased intrahepatic and circulating levels of endoglin, a TGF-β1 co-receptor, in patients with chronic hepatitis C virus infection: Relationship to histological and serum markers of hepatic fibrosis. J. Viral Hepat. 2006, 13, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Meurer, S.K.; Tihaa, L.; Borkham-Kamphorst, E.; Weiskirchen, R. Expression and functional analysis of endoglin in isolated liver cells and its involvement in fibrogenic Smad signalling. Cell. Signal. 2011, 23, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Wiercinska, E.; Wickert, L.; Denecke, B.; Said, H.M.; Hamzavi, J.; Gressner, A.M.; Thorikay, M.; ten Dijke, P.; Mertens, P.R.; Breitkopf, K.; et al. Id1 is a critical mediator in TGF-β-induced transdifferentiation of rat hepatic stellate cells. Hepatology 2006, 43, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Abreu, J.G.; Ketpura, N.I.; Reversade, B.; De Robertis, E.M. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat. Cell Biol. 2002, 4, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Goldschmeding, R. Bone morphogenetic protein-7 and connective tissue growth factor: Novel targets for treatment of renal fibrosis? Pharm. Res. 2008, 25, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Lahme, B.; Siluschek, M.; Rehbein, K.; Weiskirchen, R.; Gressner, A.M. Connective tissue growth factor is a Smad2 regulated amplifier of transforming growth factor beta actions in hepatocytes—But without modulating bone morphogenetic protein 7 signaling. Hepatology 2009, 49, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Guimei, M.; Baddour, N.; Elkaffash, D.; Abdou, L.; Taher, Y. Gremlin in the pathogenesis of hepatocellular carcinoma complicating chronic hepatitis C: An immunohistochemical and PCR study of human liver biopsies. BMC Res. Notes 2012, 5, 390. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, S.L.; Lu, X.J.; Shen, C.Y.; Liu, Y.; Chen, Y.P. Bone morphogenetic protein 7 suppresses the progression of hepatic fibrosis and regulates the expression of gremlin and transforming growth factor β1. Mol. Med. Rep. 2012, 6, 246–252. [Google Scholar] [PubMed]
- Zhang, Y.Q.; Wan, L.Y.; He, X.M.; Ni, Y.R.; Wang, C.; Liu, C.B.; Wu, J.F. Gremlin1 Accelerates Hepatic Stellate Cell Activation Through Upregulation of TGF-Β Expression. DNA Cell Biol. 2017, 36, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Soofi, A.; Wolf, K.I.; Ranghini, E.J.; Amin, M.A.; Dressler, G.R. The kielin/chordin-like protein KCP attenuates nonalcoholic fatty liver disease in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G587–G598. [Google Scholar] [CrossRef] [PubMed]
- Patella, S.; Phillips, D.J.; Tchongue, J.; de Kretser, D.M.; Sievert, W. Follistatin attenuates early liver fibrosis: Effects on hepatic stellate cell activation and hepatocyte apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G137–G144. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, M.; Shimada, M.; Morine, Y.; Yoshizumi, T.; Imura, S.; Ikegami, T.; Mori, H.; Arakawa, Y. Beneficial effects of follistatin in hepatic ischemia-reperfusion injuries in rats. Dig. Dis. Sci. 2011, 56, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, J.; Neumeier, M.; Bauer, S.; Weiss, T.S.; Eisinger, K.; Walter, R.; Dorn, C.; Hellerbrand, C.; Schaffler, A.; Buechler, C. Adiponectin induces the transforming growth factor decoy receptor BAMBI in human hepatocytes. FEBS Lett. 2011, 585, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; De Minicis, S.; Osterreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Barbara, N.P.; Wrana, J.L.; Letarte, M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-β superfamily. J. Biol. Chem. 1999, 274, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, C.; Lopez-Novoa, J.M.; Quintanilla, M. The emerging role of TGF-β superfamily coreceptors in cancer. Biochim. Biophys. Acta 2009, 1792, 954–973. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Bokhove, M.; Croci, R.; Zamora-Caballero, S.; Han, L.; Letarte, M.; de Sanctis, D.; Jovine, L. Structural Basis of the Human Endoglin-BMP9 Interaction: Insights into BMP Signaling and HHT1. Cell Rep. 2017, 19, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Felix, J.M.; Oujo, B.; Lopez-Novoa, J.M. The role of endoglin in kidney fibrosis. Expert Rev. Mol. Med. 2014, 16, e18. [Google Scholar] [CrossRef] [PubMed]
- Meurer, S.K.; Alsamman, M.; Sahin, H.; Wasmuth, H.E.; Kisseleva, T.; Brenner, D.A.; Trautwein, C.; Weiskirchen, R.; Scholten, D. Overexpression of endoglin modulates TGF-β1-signalling pathways in a novel immortalized mouse hepatic stellate cell line. PLoS ONE 2013, 8, e56116. [Google Scholar] [CrossRef] [PubMed]
- Scherner, O.; Meurer, S.K.; Tihaa, L.; Gressner, A.M.; Weiskirchen, R. Endoglin differentially modulates antagonistic transforming growth factor-β1 and BMP-7 signaling. J. Biol. Chem. 2007, 282, 13934–13943. [Google Scholar] [CrossRef] [PubMed]
- Alt, A.; Miguel-Romero, L.; Donderis, J.; Aristorena, M.; Blanco, F.J.; Round, A.; Rubio, V.; Bernabeu, C.; Marina, A. Structural and functional insights into endoglin ligand recognition and binding. PLoS ONE 2012, 7, e29948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharpfenecker, M.; van Dinther, M.; Liu, Z.; van Bezooijen, R.L.; Zhao, Q.; Pukac, L.; Lowik, C.W.; ten Dijke, P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J. Cell Sci. 2007, 120, 964–972. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Mallet, C.; Mazerbourg, S.; Feige, J.J.; Bailly, S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 2007, 109, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Conley, B.; Romero, D.; Tweedie, E.; O’Neill, C.; Pinz, I.; Brogan, L.; Lindner, V.; Liaw, L.; Vary, C.P. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood 2012, 120, 4263–4273. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Tweedie, E.; Conley, B.; Ames, J.; FitzSimons, M.; Brooks, P.; Liaw, L.; Vary, C.P. BMP9 crosstalk with the hippo pathway regulates endothelial cell matricellular and chemokine responses. PLoS ONE 2015, 10, e0122892. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem. Cell Biol. 2003, 81, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Lahme, B.; Demirci, I.; Gressner, A.M.; Weiskirchen, R. Differential effects of TGF-β on connective tissue growth factor (CTGF/CCN2) expression in hepatic stellate cells and hepatocytes. J. Hepatol. 2007, 47, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.L.; Ciuclan, L.; Liu, Y.; Hamzavi, J.; Godoy, P.; Gaitantzi, H.; Kanzler, S.; Heuchel, R.; Ueberham, U.; Gebhardt, R.; et al. Profibrogenic transforming growth factor-β/activin receptor-like kinase 5 signaling via connective tissue growth factor expression in hepatocytes. Hepatology 2007, 46, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Guanabens, N.; Ruiz-Gaspa, S.; Gifre, L.; Miquel, R.; Peris, P.; Monegal, A.; Dubrueil, M.; Arias, A.; Pares, A. Sclerostin expression in bile ducts of patients with chronic cholestasis may influence the bone disease in primary biliary cirrhosis. J. Bone Miner. Res. 2016, 31, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Kountouras, J.; Makras, P.; Papatheodorou, A.; Kokkoris, P.; Sakellariou, G.T.; Terpos, E. Circulating sclerostin and Dickkopf-1 levels in patients with nonalcoholic fatty liver disease. J. Bone Miner. Metab. 2016, 34, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Larrain, J.; Bachiller, D.; Lu, B.; Agius, E.; Piccolo, S.; De Robertis, E.M. BMP-binding modules in chordin: A model for signalling regulation in the extracellular space. Development 2000, 127, 821–830. [Google Scholar] [PubMed]
- Lin, J.; Patel, S.R.; Cheng, X.; Cho, E.A.; Levitan, I.; Ullenbruch, M.; Phan, S.H.; Park, J.M.; Dressler, G.R. Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat. Med. 2005, 11, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Patel, S.R.; Wang, M.; Dressler, G.R. The cysteine-rich domain protein KCP is a suppressor of transforming growth factor β/activin signaling in renal epithelia. Mol. Cell. Biol. 2006, 26, 4577–4585. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P.; de Kretser, D.M. The activins and their binding protein, follistatin-Diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine Growth Factor Rev. 2013, 24, 285–295. [Google Scholar] [CrossRef] [PubMed]
- De Kretser, D.M.; O’Hehir, R.E.; Hardy, C.L.; Hedger, M.P. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol. Cell. Endocrinol. 2012, 359, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kreidl, E.; Ozturk, D.; Metzner, T.; Berger, W.; Grusch, M. Activins and follistatins: Emerging roles in liver physiology and cancer. World J. Hepatol. 2009, 1, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Ooe, H.; Chen, Q.; Kon, J.; Sasaki, K.; Miyoshi, H.; Ichinohe, N.; Tanimizu, N.; Mitaka, T. Proliferation of rat small hepatocytes requires follistatin expression. J. Cell. Physiol. 2012, 227, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Beale, G.; Chattopadhyay, D.; Gray, J.; Stewart, S.; Hudson, M.; Day, C.; Trerotoli, P.; Giannelli, G.; Manas, D.; Reeves, H. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer 2008, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, A.; Haukeland, J.W.; Dahl, T.B.; Bjoro, K.; Gladhaug, I.P.; Berge, C.; Damas, J.K.; Haaland, T.; Loberg, E.M.; Linnestad, P.; et al. A complex role of activin A in non-alcoholic fatty liver disease. Am. J. Gastroenterol. 2009, 104, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, X.; Yang, L.; Kisseleva, T.; Brenner, D.A.; Seki, E. Transcriptional repression of the transforming growth factor beta (TGF-β) Pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) by Nuclear Factor kappaB (NF-kappaB) p50 enhances TGF-β signaling in hepatic stellate cells. J. Biol. Chem. 2014, 289, 7082–7091. [Google Scholar] [CrossRef] [PubMed]
- Teratani, T.; Tomita, K.; Suzuki, T.; Oshikawa, T.; Yokoyama, H.; Shimamura, K.; Tominaga, S.; Hiroi, S.; Irie, R.; Okada, Y.; et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology 2012, 142, 152–164.e10. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Teratani, T.; Suzuki, T.; Shimizu, M.; Sato, H.; Narimatsu, K.; Okada, Y.; Kurihara, C.; Irie, R.; Yokoyama, H.; et al. Free cholesterol accumulation in hepatic stellate cells: Mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology 2014, 59, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Teratani, T.; Suzuki, T.; Shimizu, M.; Sato, H.; Narimatsu, K.; Usui, S.; Furuhashi, H.; Kimura, A.; Nishiyama, K.; et al. Acyl-CoA:cholesterol acyltransferase 1 mediates liver fibrosis by regulating free cholesterol accumulation in hepatic stellate cells. J. Hepatol. 2014, 61, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Chang, H.M.; Cheng, J.C.; Klausen, C.; Chu, G.; Leung, P.C.K.; Yang, G. SMAD1/5 mediates bone morphogenetic protein 2-induced up-regulation of BAMBI expression in human granulosa-lutein cells. Cell. Signal. 2017, 37, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hutley, L.J.; Webster, J.A.; Kim, Y.H.; Liu, D.F.; Newell, F.S.; Widberg, C.H.; Bachmann, A.; Turner, N.; Schmitz-Peiffer, C.; et al. Identification of BMP and activin membrane-bound inhibitor (BAMBI) as a potent negative regulator of adipogenesis and modulator of autocrine/paracrine adipogenic factors. Diabetes 2012, 61, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Gaynor, K.U.; Rowan, S.C.; Walsh, S.M.; Fabre, A.; Boylan, J.; Keane, M.P.; McLoughlin, P. Altered expression of bone morphogenetic protein accessory proteins in murine and human pulmonary fibrosis. Am. J. Pathol. 2016, 186, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Lowery, J.W.; Rosen, V. Bone morphogenetic protein-based therapeutic approaches. Cold Spring Harb. Perspect. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ciuclan, L.; Sheppard, K.; Dong, L.; Sutton, D.; Duggan, N.; Hussey, M.; Simmons, J.; Morrell, N.W.; Jarai, G.; Edwards, M.; et al. Treatment with anti-gremlin 1 antibody ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension in mice. Am. J. Pathol. 2013, 183, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Gressner, A.M. Connective tissue growth factor: A fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008, 28, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.H.; Brazil, D.P. Bone morphogenetic proteins and their antagonists: Current and emerging clinical uses. Br. J. Pharmacol. 2014, 171, 3620–3632. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; LeBleu, V.S.; Bosukonda, D.; Keck, P.; Taduri, G.; Bechtel, W.; Okada, H.; Carlson, W., Jr.; Bey, P.; Rusckowski, M.; et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat. Med. 2012, 18, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Potential Effect on Liver Fibrosis | Regulatory Effect on BMP Signaling in Liver Fibrosis | References | |
|---|---|---|---|
| Endoglin | Profibrotic | Not determined. Indirect data would support a stimulatory effect on BMP signaling | [132,133,134] |
| CTGF | Profibrotic | CTGF does not interfere with BMP7 signaling BMP7 inhibits CTGF expression | [135,136,137] |
| Gremlin | Profibrotic | It inhibits BMP7 antifibrotic signaling | [138,139,140] |
| KCP | Antifibrotic | Not determined | [141] |
| Follitastin | Antifibrotic | Not determined | [142,143] |
| Bambi | Antifibrotic | Not determined | [144,145] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, B.; Addante, A.; Sánchez, A. BMP Signalling at the Crossroad of Liver Fibrosis and Regeneration. Int. J. Mol. Sci. 2018, 19, 39. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010039
Herrera B, Addante A, Sánchez A. BMP Signalling at the Crossroad of Liver Fibrosis and Regeneration. International Journal of Molecular Sciences. 2018; 19(1):39. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010039
Chicago/Turabian StyleHerrera, Blanca, Annalisa Addante, and Aránzazu Sánchez. 2018. "BMP Signalling at the Crossroad of Liver Fibrosis and Regeneration" International Journal of Molecular Sciences 19, no. 1: 39. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010039