Role of Stem Cells in Pathophysiology and Therapy of Spondyloarthropathies—New Therapeutic Possibilities?
Abstract
:1. Introduction
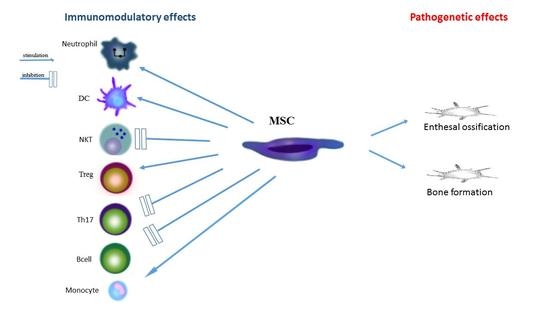
2. The Role of Mesenchymal Stromal Cells in the Inflammatory Process and in the Pathogenesis of Spondyloarthropathies
2.1. Origin of Stromal Cells
2.2. The Role of Toll-Like Receptors in Activity of Stem Cells
2.3. Stem Cells at an Early Phase of Inflammation
2.4. Monocytes and Macrophages
2.5. Dendritic Cells
2.6. Neutrophils
2.7. NK Cells
2.8. T Cells
2.9. B Cells
3. The Role of Stem Cells of Irregular Ossification in Spondyloarthropathy
4. The Role of MSC in the Treatment of Spondyloarthropathies
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rutwaleit, M. New approaches to diagnosis and classification of axial and peripheral spondyloarthritis. Curr. Opin. Rheumatol. 2010, 22, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Callhoff, J.; Sieper, J.; Weiß, A.; Zink, A.; Listing, J. Efficacy of TNF-α blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: A meta-analysis. Ann. Rheum. Dis. 2015, 74, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyy, D.; Hermann, K.G.; Callhoff, J.; Listing, J.; Sieper, J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: Results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann. Rheum. Dis. 2014, 73, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Baraliakos, X.; Braun, J.; Sieper, J.; Emery, P.; van der Heijde, D.; McInnes, I.; van Laar, J.M.; Landewé, R.; Wordsworth, P.; et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: A randomised, double-blind, placebocontrolled trial. Lancet 2013, 382, 1705–1713. [Google Scholar] [CrossRef]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Colter, D.C.; Sekiya, I.; Prockop, D.J. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc. Natl. Acad. Sci. USA 2001, 98, 7841–7845. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Kurtz, A.; Barutcu, N.; Bodo, J.; Thiel, A.; Dong, J. Concerted regulation of CD34 and CD105 accompanies mesenchymal stromal cell derivation from human adventitial stromal cell. Stem Cells Dev. 2013, 22, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.D.; Wagner, W.; Franke, W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy 2008, 10, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Girlovanu, M.; Susman, S.; Soritau, O.; Rus-Ciuca, D.; Melincovici, C.; Constantin, A.M.; Mihu, C.M. Stem cells—Biological update and cell therapy progress. Clujul Med. 2015, 88, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I. Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: Their comparative efficacies and synergistic effects. J. Biomed. Mater. Res. A 2017, 105, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013, 31, 285–316. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. Concise review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells 2013, 31, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Delarosa, O.; Dalemans, W.; Lombardo, E. Toll-like receptors as modulators of mesenchymal stem cells. Front. Immunol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed]
- Raicevic, G.; Rouas, R.; Najar, M.; Stordeur, P.; Boufker, H.I.; Bron, D.; Martiat, P.; Goldman, M.; Nevessignsky, M.T.; Lagneaux, L. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum. Immunol. 2010, 71, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Mo, I.F.; Yip, K.H.; Chan, W.K.; Law, H.K.; Lau, Y.L.; Chan, G.C. Prolonged exposure to bacterial toxins downregulated expression of toll-like receptors in mesenchymal stromal cell-derived osteoprogenitors. BMC Cell Biol. 2008, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Heuschen, G.; Leowardi, C.; Hinz, U.; Autschbach, F.; Stallmach, A.; Herfarth, C.; Heuschen, U.A. Differential expression of toll-like receptor 3 and 5 in ileal pouch mucosa of ulcerative colitis patients. Int. J. Colorectal Dis. 2007, 22, 293–301. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, L.; Vandooren, B.; Kruithof, E.; De Keyser, F.; Veys, E.M.; Baeten, D. Tumor necrosis factor alpha blockade treatment down-modulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondylarthropathy. Arthritis Rheum. 2005, 52, 2146–2158. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Liang, Y.; Zhu, Y.; Li, C.; Zhang, L.Z.; Zeng, X.M.; Zhong, R.Q. Increased expression of Toll-like receptor 4 in peripheral blood leucocytes and serum levels of some cytokines in patients with ankylosing spondylitis. Clin. Exp. Immunol. 2007, 149, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Candia, L.; Marquez, J.; Hernandez, C.; Zea, A.H.; Espinoza, L.R. Toll-like receptor-2 expression is upregulated in antigen-presenting cells from patients with psoriatic arthritis: A pathogenic role for innate immunity. J. Rheumatol. 2007, 34, 374–379. [Google Scholar] [PubMed]
- Myles, A.; Aggarwal, A. Expression of Toll-like receptors 2 and 4 is increased in peripheral blood and synovial fluid monocytes of patients with enthesitis-related arthritis subtype of juvenile idiopathic arthritis. Rheumatology 2011, 50, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Jakob, M.; Hemeda, H.; Bruderek, K.; Janeschik, S.; Bootz, F.; Lang, S. Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J. Leukoc. Biol. 2010, 88, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Cassatella, M.A.; Mosna, F.; Micheletti, A.; Lisi, V.; Tamassia, N.; Cont, C.; Calzetti, F.; Pelletier, M.; Pizzolo, G.; Krampera, M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells 2011, 29, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Jia, T.; Mendez-Ferrer, S.; Hohl, T.M.; Serbina, N.V.; Lipuma, L.; Leiner, I.; Li, M.O.; Frenette, P.S.; Pamer, E.G. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 2011, 34, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Abomaray, F.M.; Fatani, A.S.; Chamley, L.W.; Knawy, B.A. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. 2013, 9, 620–641. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pubmed/23812784 (accessed on 25 November 2017). [CrossRef] [PubMed]
- Cho, D.I.; Kim, M.R.; Jeong, H.Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef] [PubMed]
- Dayan, V.; Yannarelli, G.; Billia, F.; Filomeno, P.; Wang, X.H.; Davies, J.E.; Keating, A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res. Cardiol. 2011, 106, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Hoogduijn, M.J. Mesenchymal stem cell-educated macrophages. Transp. Res. 2012, 1, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, W.; Tao, C.; Sun, P.; Yang, Z.; Xu, W. M2 polarization of monocytes in ankylosing spondylitis and relationship with inflammation and structural damage. APMIS 2017. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Alessandro, R.; Rizzo, A.; Accardo-Palumbo, A.; Raimondo, S.; Raiata, F.; Guggino, G.; Giardina, A.; De Leo, G.; Sireci, G.; et al. Macrophage phenotype in the subclinical gut inflammation of patients with ankylosing spondylitis. Rheumatology 2014, 53, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Van Kuijk, A.W.; Reinders-Blankert, P.; Smeets, T.J.; Dijkmans, B.A.; Tak, P.P. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: Implications for treatment. Ann. Rheum Dis. 2006, 65, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.Y.; Ju, J.H.; Park, S.H.; Kim, H.Y. The paradoxical effects of TNF inhibitors on bone mineral density and radiographic progression in patients with ankylosing spondylitis. Rheumatology 2013, 52, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D.; et al. Induction of Osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillon, S.M.; Rogers, L.M.; Howe, R.; Hostetler, L.A.; Buhrman, J.; McCarter, M.D.; Wilson, C.C. Human intestinal lamina propria CD1c+ dendritic cells display an activated phenotype at steady state and produce IL-23 in response to TLR7/8 stimulation. J. Immunol. 2010, 184, 6612–6621. [Google Scholar] [CrossRef] [PubMed]
- DeLay, M.L.; Turner, M.J.; Klenk, E.I.; Smith, J.A.; Sowders, D.P.; Colbert, R.A. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009, 60, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Utriainen, L.; Firmin, D.; Wright, P.; Cerovic, V.; Breban, M.; McInnes, I.; Milling, S. Expression of HLA-B27 causes loss of migratory dendritic cells in a rat model of spondyloarthritis. Arthritis Rheum. 2012, 64, 3199–3209. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, J.P.; Joyce-Shaikh, B.; Turner, S.P.; Chao, C.C.; Sathe, M.; Grein, J.; Gorman, D.M.; Bowman, E.P.; McClanahan, T.K.; Yearley, J.H.; et al. IL-23 induces spondyloarthropathy by acting on ROR-γt(+)CD3(+)CD4(-)CD8(-) entheseal resident T cells. Nat. Med. 2012, 18, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Kruisselbrink, A.B.; Lurvink, E.; Willemze, R.; Fibbe, W.E. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J. Immunol. 2006, 177, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ge, W.; Li, C.; You, S.; Liao, L.; Han, Q.; Deng, W.; Zhao, R.C. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004, 13, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Zhang, Y.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Morbelli, S.; Morando, S.; Massollo, M.; Marini, C.; Bertoni, A.; Frassoni, F.; Bartolomé, S.T.; Sambuceti, G.; Traggiai, E.; et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17384–17389. [Google Scholar] [CrossRef] [PubMed]
- Favaro, E.; Carpanetto, A.; Caorsi, C.; Giovarelli, M.; Angelini, C.; Cavallo-Perin, P.; Tetta, C.; Camussi, G.; Zanone, M.M. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 2016, 59, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.; Maier, R.; Wu, P.; Scheer, R.; Hempfing, A.; Kayser, R.; Thiel, A.; Radbruch, A.; Loddenkemper, C.; Sieper, J. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 2011, 13, R95. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Vidyadaran, S.; George, E.; Ramasamy, R. Human mesenchymal stem cells protect neutrophils from serum-deprived cell death. Cell Biol. Int. 2011, 35, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Bianchi, G.; Bertolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 2008, 26, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Zvyagin, I.V.; Mamedov, I.Z.; Britanova, O.V.; Staroverov, D.B.; Nasonov, E.L.; Bochkova, A.G.; Chkalina, A.V.; Kotlobay, A.A.; Korostin, D.O.; Rebrikov, D.V.; et al. Contribution of functional KIR3DL1 to ankylosing spondylitis. Cell. Mol. Immunol. 2010, 7, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Prigione, I.; Benvenuto, F.; Bocca, P.; Battistini, L.; Uccelli, A.; Pistoia, V. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells 2009, 27, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Galipeau, J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr. Mol. Med. 2013, 13, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, S.; Pene, J.; Torcy-Moquet, G.; Jorgensen, C.; Yssel, H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, S.; Qu, X.; Ge, C.; Cheng, K.; Zhao, R.C. Mesenchymal stem cells inhibit Th17 cells differentiation via IFN-γ-mediated SOCS3 activation. Immunol. Res. 2015, 61, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Rafei, M.; Campeau, P.M.; Aguilar-Mahecha, A.; Buchanan, M.; Williams, P.; Birman, E.; Yuan, S.; Young, Y.K.; Boivin, M.N.; Forner, K.; et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J. Immunol. 2009, 182, 5994–6002. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Zhu, J.; Lu, S.H.; Zhang, J.L.; Chen, X.; Du, L.X.; Yang, Z.G.; Song, Y.K.; Wu, D.Y.; Liu, B.; et al. Inhibitory effect of human umbilical cord-derived mesenchymal stem cells on interleukin-17 production in peripheral blood T cells from spondyloarthritis patients. Zhongguo Shi Yan Xue Ye Za Zhi 2013, 21, 455–459. [Google Scholar] [CrossRef]
- Shen, H.; Goodall, J.C.; Hill Gaston, J.S. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009, 60, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Limón-Camacho, L.; Vargas-Rojas, M.I.; Vázquez-Mellado, J.; Casasola-Vargas, J.; Moctezuma, J.F.; Burgos-Vargas, R.; Llorente, L. In vivo peripheral blood proinflammatory T cells in patients with ankylosing spondylitis. J. Rheumatol. 2012, 39, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Kurte, M.; Bravo-Alegría, J.; Contreras, R.; Nova-Lamperti, E.; Tejedor, G.; Noël, D.; Jorgensen, C.; Figueroa, F.; Djouad, F.; et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Obermajer, N.; Popp, F.C.; Soeder, Y.; Haarer, J.; Geissler, E.K.; Schlitt, H.J.; Dahlke, M.H. Conversion of Th17 into IL-17A(neg) regulatory T cells: A novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. J. Immunol. 2014, 193, 4988–4999. [Google Scholar] [CrossRef] [PubMed]
- Xueyi, L.; Lina, C.; Zhenbiao, W.; Qing, H.; Qiang, L.; Zhu, P. Levels of circulating Th17 cells and regulatory T cells in ankylosing spondylitis patients with an inadequate response to anti-TNF-alpha therapy. J. Clin. Immunol. 2013, 33, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.; Wu, P.; Scheer, R.; Kedor, C.; Sawitzki, B.; Thiel, A.; Radbruch, A.; Sieper, J.; Syrbe, U. Synovial and peripheral blood CD4+FoxP3+ T cells in spondyloarthritis. J. Rheumatol. 2011, 38, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zheng, M.; Zhang, K.; Yang, F.; Zhang, X.; Han, Q.; Chen, Z.N.; Zhu, P. Functional defects in CD4+ CD25high FoxP3+ regulatory cells in ankylosing spondylitis. Sci. Rep. 2016, 6, 37559. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ren, M.; Yang, R.; Liang, X.; Ma, Y.; Tang, Y.; Huang, L.; Ye, J.; Chen, K.; Wanget, P.; et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res. Ther. 2011, 13, R29. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25Highforkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Schrama, C.L.M.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes towards anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- LeMaoult, J.; Caumartin, J.; Daouya, M.; Favier, B.; Le Rond, S.; Gonzalez, A.; Carosella, E.D. Immune regulation by pretenders: Cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood 2007, 109, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E.; Randall, T.D. Effector and regulatory B cells: Modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 2010, 10, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Nova-Lamperti, E.; Fanelli, G.; Becker, P.D.; Chana, P.; Elgueta, R.; Dodd, P.C.; Lord, G.M.; Lombardi, G.; Hernandez-Fuentesa, M.P. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4(+)T-cell responses. Sci. Rep. 2016, 6, 20044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantaert, T.; Doorenspleet, M.E.; Francosalinas, G.; Paramarta, J.E.; Klarenbeek, P.L.; Tiersma, Y.; van der Loos, C.M.; De Vries, N.; Tak, P.P.; Baeten, D.L. Increased numbers of CD5+ B lymphocytes with a regulatory phenotype in spondylarthritis. Arthritis Rheum. 2012, 64, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Caro, M.B.; de Miguel, E.; Peiteado, D.; Plasencia-Rodríguez, C.; Villalba, A.; Monjo-Henry, I.; Puig-Kröger, A.; Sánchez-Mateos, P.; Martín-Mola, E.; Miranda-Carús, M.E. Increased frequency of circulating CD19+CD24hiCD38hi B cells with regulatory capacity in patients with Ankylosing spondylitis (AS) naïve for biological agents. PLoS ONE 2017, 12, e0180726. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Yi, T.G.; Lee, H.J.; Kim, S.N.; Park, S.; Jeon, M.S.; Song, S.U. Mesenchymal stem cells infected with Mycoplasma arginini secrete complement C3 to regulate immunoglobulin production in b lymphocytes. Cell Death Dis. 2014, 5, e1192. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, M.; Yuan, Y.; Wang, Z.Z.; Jiang, H.; Chen, T. Priming of Toll-like receptor 4 pathway in mesenchymal stem cells increases expression of B cell activating factor. Biochem. Biophys. Res. Commun. 2014, 448, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, C.; Quade-Lyssy, P.; Radeke, H.H.; Henschler, R.; Konigs, C.; Kohl, U.; Seifried, E.; Schüttrumpf, J. Galectin-9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2014, 23, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.R.; Yang, Z.X.; Han, Z.B.; Meng, L.; Liang, L.; Feng, X.M.; Yang, S.G.; Chi, Y.; Chen, D.D.; Wang, Y.W.; et al. Mesenchymal stem cells support proliferation and terminal differentiation of B cells. Cell Physiol. Biochem. 2012, 30, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ghali, O.; Lencel, P.; Broux, O.; Chauveau, C.; Devedjian, J.C.; Hardouin, P.; Magne, D. TNFα and IL1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009, 84, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Briolay, A.; Lencel, P.; Bessueille, L.; Caverzasio, J.; Buchet, R.; Magne, D. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-α in human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2013, 430, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Kurth, T.B.; Augello, A. Mesenchymal stem cells from development to postnatal joint homeostasis, aging, and disease. Birth Defects Res. C. Embryo Today 2010, 90, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Nourissat, G.; Diop, A.; Maurel, N.; Salvat, C.; Dumont, S.; Pigenet, A.; Gosset, M.; Houard, X.; Berenbaum, F. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS ONE 2010, 5, e12248. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.F.; Lui, P.P.; Ni, M.; Chan, L.S.; Lee, Y.W.; Chan, K.M. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J. Orthop. Res. 2011, 29, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Guerra, G. Ca2+ Signalling in endothelial progenitor cells: Friend or foe? J. Cell Physiol. 2016, 231, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Ronco, V.; Potenza, D.M.; Denti, F.; Vullo, S.; Gagliano, G.; Tognolina, M.; Guerra, G.; Pinton, P.; Genazzani, A.A.; Mapelli, L.; et al. A novel Ca2+-mediated cross-talk between endoplasmic reticulum and acidic organelles: Implications for NAADP-dependent Ca2+ signaling. Cell Calcium 2015, 57, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Sun, J.; Lu, S.; Qi, Y.X.; Wang, Y. Prolonged mechanical stretch initiates intracellular calcium oscillations in human mesenchymal stem cells. PLoS ONE 2014, 9, e109378. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Joo, C.; Seong, J.; Vafabakhsh, R.; Botvinick, E.L.; Berns, M.W.; Palmer, A.E.; Wang, N.; Ha, T.; Jakobsson, E.; et al. Distinct mechanisms regulating mechanical force-induced Ca2+ signals at the plasma membrane and the ER in human MSCs. eLife 2015, 4, e04876. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, P.; Li, Y.; Deng, W.; Zhang, X.; Su, H.; Li, D.; Wu, Y.; Shen, H. Imbalance between BMP2 and Noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol. 2016, 68, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Bassi, E.J.; Moraes-Vieira, P.M.; Moreira-Sa, C.S.; Almeida, D.C.; Vieira, L.M.; Cunha, C.S.; Hiyane, M.I.; Basso, A.S.; Pacheco-Silva, A.; Câmara, N.O. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 2012, 61, 2534–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, P.; Souza-Moreira, L.; Morell, M.; Caro, M.; O’Valle, F.; Gonzalez-Rey, E.; Delgado, M. Adipose-derived mesenchymal stromal cells induce mmunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 2013, 62, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, R.H.; Yu, J.M.; Ko, J.H.; Lee, H.J.; Ko, A.Y.; Roddy, G.W.; Prockop, D.J. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol. Ther. 2012, 20, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F.; et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Mathias, L.J.; Khong, S.M.; Spyroglou, L.; Payne, N.L.; Siatskas, C.; Thorburn, A.N.; Boyd, R.L.; Heng, T.S. Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. J. Immunol. 2013, 191, 5914–5924. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, L.; Sarukhan, A.; Dander, E.; Castor, M.; Cibella, J.; Soldani, C.; Trovato, A.E.; Ploia, C.; Luca, G.; Calvitti, M.; et al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia 2013, 27, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Swart, J.F.; Wulffraat, N.M. Mesenchymal stromal cells for treatment of arthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Wyles, C.C.; Houdek, M.T.; Behfar, A.; Sierra, R.S. Mesenchymal stem cell therapy for osteoarthritis: Current perspectives. Stem Cells Cloning 2015, 8, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hinterberger, W.; Hinterberger-Fischer, M.; Marmont, A. Clinically demonstrable anti-autoimmunity mediated by allogeneic immune cells favorably affects outcome after stem cell transplantation in human autoimmune diseases. Bone Marrow Transplant. 2002, 30, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Slavin, S.; Nagler, A.; Varadi, G.; Or, R. Graft vs autoimmunity following allogeneic non-myeloablative blood stem cell transplantation in a patient with chronic myelogenous leukaemia and severe systemic psoriasis and psoriatic polyarthritis. Exp. Hematol. 2000, 28, 853–857. [Google Scholar] [CrossRef]
- Woods, A.C.; Mant, M.J. Amelioration of severe psoriasis with psoriatic arthritis for 20 years after allogeneic haematopoietic stem cell transplantation. Ann. Rheum. Dis. 2006, 65, 697. [Google Scholar] [CrossRef] [PubMed]
- Braiteh, F.; Hymes, S.R.; Giralt, S.A.; Jones, R. Complete remission of psoriasis after autologous hematopoietic stem-cell transplantation for multiple myeloma. J. Clin. Oncol. 2008, 26, 4511–4513. [Google Scholar] [CrossRef] [PubMed]
- Jantumen, E.; Myllykangas-Luosujärvi, R.; Kaipiainen-Seppänen, O.; Nousiainen, T. Autologous stem cell transplantation in a lymphoma patient with a long history of ankylosing spondylitis. Rheumatology 2000, 39, 563–564. [Google Scholar] [CrossRef]
- Yang, H.K.; Moon, S.J.; Shin, J.H.; Kwok, S.K.; Park, K.S.; Park, S.H.; Kim, H.Y.; Ju, J.H. Regression of syndesmophyte after bone marrow transplantation for acute myeloid leukemia in a patient with ankylosing spondylitis: A case report. J. Med. Case Rep. 2012, 6, 250. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC3459693/ (accessed on 25 November 2017). [CrossRef] [PubMed]
- Britanova, O.V.; Bochkova, A.G.; Staroverov, D.B.; Feforenko, D.A.; Bolotin, D.A.; Memedove, I.Z.; Turchaaninova, M.A.; Putintseva, E.V.; Kotlobay, A.A.; Lukyanov, S.; et al. First autologous hematopoietic SCT for ankylosing spondylitis: A case report and clues to understanding the therapy. Bone Marrow Transplant. 2012, 47, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Huang, L.; Yang, J.; Yang, R.; Deng, W.; Liang, B.; Dai, L.; Meng, Q.; Gao, L.; et al. Effects and safety of allogenic mesenchymal stem cells intravenous infusion in active ankylosing spondylitis patients who failed NSAIDs: A 20 week clinical trial. Cell Transplant. 2014, 23, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Safety and Efficacy Study of Umbilical Cord/Placenta-Derived Mesenchymal Stem Cells to Treat Ankylosing Spondylitis. ClinicalTrials.gov Identifier: NCT01420432. Available online: www.clinicaltrials.gov (accessed on 22 October 2017).
- ClinicalTrials.gov. A Molecule Basic Study of Early Warning of New Pathogenic Risk of Ankylosing Spondylitis. ClinicalTrial.gov Identifier: NCT01709656. Available online: www.clinicaltrials.gov (accessed on 22 October 2017).
- ClinicalTrials.gov. A Pilot Study of MSCs Infusion and Etanercept to Treat Ankylosing Spondylitis. ClinicalTrial.gov Identifier: NCT02809781. Available online: www.clinicaltrials.gov (accessed on 22 October 2017).
- Chinese Clinical Trial Registry. Clinical Study of Mesenchymal Stem Cells Transplantation in Ankylosing Spondylitis. Registration Number: ChiCTR-TRC-11001417. Available online: http://www.chictr.org.cn/showprojen.aspx?proj=8122 (accessed on 22 October 2017).


| Elements of Pathogenesis of Spondyloarthropathy | Results of Stem Cell Action |
|---|---|
| Dysregulation of TLR. Increase in expression of TLR2 and TLR 4 on mononuclear cells of peripheral blood and in articular synovial membrane [21,22,23,24]. | Acquisition of the pro-inflammatory phenotype by MSC following stimulation by TLR4 and the anti-inflammatory phenotype following stimulation by TLR3 [18,19,20]. |
| Increased production of pro-inflammatory TNF-α and IFN-γ by activated monocytes and macrophages. | Activation of MSC with TNF-α and IFN-γ boosts expression of iNOS, COX2 and IDO and favours polarisation of monocytes and macrophages to the anti-inflammatory M2 phenotype M2 [34,35,36]. |
| Increase in production of inflammatory cytokines, e.g., IL-12, IL-23, IL-6 by dendritic cells [42,43]. | Inhibition of differentiation of precursors of CD40CD1a into DC, inhibition of the ability to present antigen by DC, induction of the loss of maturity features by DC [46,48,49]. |
| Increase in local production of IL-17 in joints by neutrophils [52]. | Inhibition of apoptosis and stimulation of activity of activity of neutrophils by IL-6, IL-8 IFN-β and GM-CSF [28,54]. |
| A link between expression of activating KIR receptors on NK cells with the disease activity. Recognising of HLA B27 antigen by the KIR3DL1 receptor [55]. | Inhibition of proliferation, cytokine secretion and cytotoxicity of NK cells [56,57,58,59]. |
| The key role of Th17 cells in development of SpA [67,68] | Ability of mature Th17 to convert into Treg [69,70]. |
| Decrease in the amount of Treg. Upsetting the Treg/Th17 balance. Functional defects of CD4+CD25+FOXP3 [71,72,73,74]. | Induction of Treg proliferation. Stimulation of differentiation of CD4 towards CD4+CD25+FOXP3 [75]. |
| Ossification of entheses, formation of new bone tissue on marginal surfaces of joints [1]. | Regulation of ossification with TNAP. Increased bone formation by activation of Wnt/β-catenin pathway with Wnt5a. Ossification of entheses following stimulation of calcium channels in MSC by mechanical stimuli [89,90,97]. |
| SpA | Stem Cells | Description | Reference |
|---|---|---|---|
| Psoriatic arthritis | Allogenic blood stem cell transplantation (myeloablative) | Concomitant chronic myelogenous leukemia. Graft versus autoimmunity effect. | Slavin et al. [109] |
| Psoriatic arthritis | Allogenic hematopoetic stem cell transplantation | Concomitant aplastic anemia. Short remission with long chronic disability-free period | Woods et al. [110] |
| Psoriatic arthritis | Autologous hematopoetic stem cell transplantation (myeloablative) | Concomitant multiple myeloma. Complete remission of arthritis and skin lesions | Braiteh et al. [111] |
| Ankylosing spondylitis | Autologous hematopoetic stem cell transplantation | Concomitant lymphoma. The patient underwent chemotherapy. Clinical remission for both AS and lymphoma | Jantumen et al. [112] |
| Ankylosing spondylitis | Allogenic blood stem cell transplantation | Concomitant acute myeloid leukemia. The patient underwent chemotherapy and body irradiation. Clinical remission. Partial radiological regression of syndesophytes | Britanova et al. [114] |
| Ankylosing spondylitis | Autologus hematopoetic stem cell transplant | The first reported intentional stem cell transplant for AS. The patient underwent chemotherapy. Complete remission for AS for two-year follow up period | Yang et al. [113] |
| Ankylosing spondylitis | Allogenic mesenchymal stem cells intravenous infusion | Trial involving 31 AS patients. No adverse effects noted. Reduction of ASDAS-CRP from 3.6 ± 0.6 to 2.4 ± 0.5 at the 4th week. The percentage of ASAS 20 responders reached 77.4% | Wanga et al. [115] |
| Ankylosing spondylitis | Human umbilical cord-derived mesenchymal stem cells | Clinical trial. Phase 1. Human umbilical cord-derived MSCs at a dose of 1.0 × 106 MSC/kg, repeated after three months and DMARDs such as sulfasalazine, methotrexate, thalidomide for 12 months | Clinical Trials. gov Identifier: NCT01420432 [116] |
| Ankylosing spondylitis | Human mesenchymal stem cells | Clinical trial. human mesenchymal stem cells: 1.0 × 104-6 cells/kg, IV on day 1 of each 14–60 day cycle, 1–6 times treatment, plus NSAIDs. | ClinicalTrials.gov Identifier: NCT01709656 [117] |
| Ankylosing spondylitis | Human bone marrow-derived MSCs | Recruiting clinical trial. Phase 2. hBM-MSCs at a dose of 1.0 × 106 MSC/kg, receive infusion per week in the first 4 weeks and every two weeks in the second 8 weeks. Study Start Date: June 2016 Estimated Study Completion Date: December 2018 | ClinicalTrials.gov Identifier: NCT02809781 [118] |
| Ankylosing spondylitis | Mesenchymal stem cells | Clinical trial. Phase I/II. To observe the safety and clinical effect of MSC transplantation in AS | Clinical trial. Registration number: ChiCTR-TRC-11001417 [119] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W.; Osowski, A.; Engelgardt, P.; Wojtkiewicz, J. Role of Stem Cells in Pathophysiology and Therapy of Spondyloarthropathies—New Therapeutic Possibilities? Int. J. Mol. Sci. 2018, 19, 80. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010080
Krajewska-Włodarczyk M, Owczarczyk-Saczonek A, Placek W, Osowski A, Engelgardt P, Wojtkiewicz J. Role of Stem Cells in Pathophysiology and Therapy of Spondyloarthropathies—New Therapeutic Possibilities? International Journal of Molecular Sciences. 2018; 19(1):80. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010080
Chicago/Turabian StyleKrajewska-Włodarczyk, Magdalena, Agnieszka Owczarczyk-Saczonek, Waldemar Placek, Adam Osowski, Piotr Engelgardt, and Joanna Wojtkiewicz. 2018. "Role of Stem Cells in Pathophysiology and Therapy of Spondyloarthropathies—New Therapeutic Possibilities?" International Journal of Molecular Sciences 19, no. 1: 80. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010080