Endogenous Ovarian Angiogenesis in Polycystic Ovary Syndrome-Like Rats Induced by Low-Frequency Electro-Acupuncture: The CLARITY Three-Dimensional Approach
Abstract
:1. Introduction
2. Results
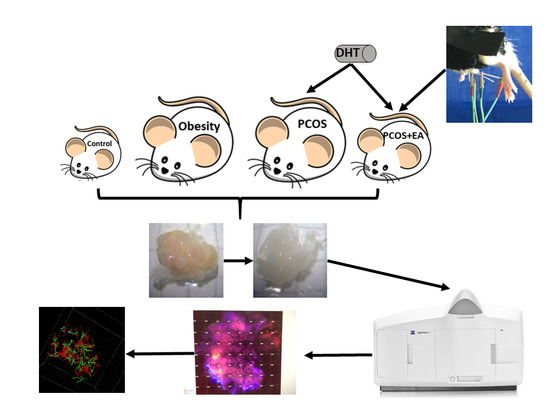
2.1. Pathological Manifestations in DHT-Induced PCOS-Like Rats and the Effect of EA
2.2. Quantitative Analysis of Follicles and CL in Ovaries Using the CLARITY Methods.
2.3. EA Promotes Angiogenesis in PCOS-Like Ovaries
2.4. EA Promotes Antral Follicle Maturation through Their Surrounding Neovasculature
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Establishment of the PCOS-Like Rat
4.3. Low-Frequency EA
4.4. CLARITY Approach
4.5. Immunofluorescence Staining
4.6. Digital Imaging and Data Analysis
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PCOS | Polycystic ovary syndrome |
| EA | Electro-acupuncture |
| DHT | 5α-dihydrotestosterone |
| CL | Corpus luteum |
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet derived growth factor |
| TGF-β | Transforming growth factor beta |
| bFGF | Basic fibroblast growth factor |
| PECAM-1/CD31 | Platelet endothelial cell adhesion molecule 1 |
| PCNA | Proliferating cell nuclear antigen |
| DHEA | dehydroepiandrosterone |
References
- Li, R.; Zhang, Q.; Yang, D.; Li, S.; Lu, S.; Wu, X.; Wei, Z.; Song, X.; Wang, X.; Fu, S. Prevalence of polycystic ovary syndrome in women in China: A large community-based study. Hum. Reprod. 2013, 28, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Michelmore, K.F.; Balen, A.H.; Dunger, D.B.; Vessey, M.P. Polycystic ovaries and associated clinical and biochemical features in young women. Clin. Endocrinol. 2010, 51, 779–786. [Google Scholar] [CrossRef]
- Yildiz, B.O.; Bozdag, G.; Yapici, Z.; Esinler, I.; Yarali, H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum. Reprod. 2012, 27, 3067–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraretti, A.P.; Marca, A.L.; Fauser, B.C.J.M.; Tarlatzis, B.; Nargund, G.; Gianaroli, L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum. Reprod. 2011, 26, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cui, P.; Lu, X.; Hsueh, B.; Möller, B.F.; Zarnescu, Y.L.; Tomer, R.; Boerboom, D.; Carmeliet, P.; Deisseroth, K. CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions. Sci. Rep. 2017, 7, 44810. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Seifer, D.B.; Arici, A. The emerging role of angiogenic factor dysregulation in the pathogenesis of polycystic ovarian syndrome. Semin. Reprod. Med. 2015, 33, 195–207. [Google Scholar] [PubMed]
- Abbott, D.H.; Dumesic, D.A.; Franks, S. Developmental origin of polycystic ovary syndrome—A hypothesis. J. Endocrinol. 2002, 174, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.A.; Wu, M.H.; Cheng, Y.C.; Li, C.H.; Chang, F.M. Quantification of Doppler signal in polycystic ovary syndrome using three-dimensional power Doppler ultrasonography: A possible new marker for diagnosis. Hum. Reprod. 2002, 17, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Tognazzi, K.; Dvorak, H.F.; Harrist, T.J. Strong expression of kinase insert domain-containing receptor, a vascular permeability factor/vascular endothelial growth factor receptor in AIDS-associated Kaposi’s sarcoma and cutaneous angiosarcoma. Am. J. Pathol. 1996, 148, 1065–1074. [Google Scholar] [PubMed]
- Abramovich, D.; Irusta, G.; Bas, D.; Cataldi, N.I.; Parborell, F.; Tesone, M. Angiopoietins/TIE2 system and VEGF are involved in ovarian function in a DHEA rat model of polycystic ovary syndrome. Endocrinology 2012, 153, 3446. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Johansson, J.; Shao, R.; Holm, L.M.; Billig, H.; Stener-Victorin, E. Electrical and manual acupuncture stimulation affect oestrous cyclicity and neuroendocrine function in an 5α-dihydrotestosterone-induced rat polycystic ovary syndrome model. Exp. Physiol. 2012, 97, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokosar, M.; Benrick, A.; Perfilyev, A.; Nilsson, E.; Källman, T.; Ohlsson, C.; Ling, C.; Stenervictorin, E. A Single Bout of Electroacupuncture Remodels Epigenetic and Transcriptional Changes in Adipose Tissue in Polycystic Ovary Syndrome. Sci. Rep. 2018, 8, 1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhang, J.; Yang, H.; Wang, F.; Li, S. Acupoint specificity on acupuncture regulation of hypothalamic- pituitary-adrenal cortex axis function. BMC Complement. Altern. Med. 2015, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Li, Y.; Wu, X.; Hou, L.; Wu, T.; Liu, J.; Ng, E.H.Y.; Stenervictorin, E.; Legro, R.S.; Zhang, H. Acupuncture and Clomiphene Citrate for Live Birth in Polycystic Ovary Syndrome: Study Design of a Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2013, 2013, 527303. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Armour, M.; Ee, C. Complementary Therapies and Medicines and Reproductive Medicine. Semin. Reprod. Med. 2016, 34, 067–073. [Google Scholar]
- Stener-Victorin, E.; Jedel, E.; Mannerås, L. Acupuncture in Polycystic Ovary Syndrome: Current Experimental and Clinical Evidence. J. Neuroendocr. 2010, 20, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.K.; Stenervictorin, E.; Kuang, H.Y.; Ma, H.L.; Gao, J.S.; Xie, L.Z.; Hou, L.H.; Hu, Z.X.; Shao, X.G.; Ge, J. Effect of Acupuncture and Clomiphene in Chinese Women With Polycystic Ovary Syndrome: A Randomized Clinical Trial. JAMA 2017, 317, 2502–2514. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Johansson, J.; Shao, R.; Mannerås, L.; Fernandez-Rodriguez, J.; Billig, H.; Stener-Victorin, E. Hypothalamic neuroendocrine functions in rats with dihydrotestosterone-induced polycystic ovary syndrome: Effects of low-frequency electro-acupuncture. PLoS ONE 2009, 4, e6638. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Kobayashi, R.; Kurosawa, M. Ovarian blood flow responses to electro-acupuncture stimulation at different frequencies and intensities in anaesthetized rats. Auton. Neurosci. Basic Clin. 2003, 108, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.L.; Kim, S.C.; Zhang, J.; Liu, H.F.; Lee, B.H.; Jang, E.Y.; Lee, C.W.; Cho, I.J.; An, W.G.; Yang, C.H. Hypothalamic Norepinephrine Mediates Acupunctural Effects on Hypothalamic-Pituitary-Adrenal Axis During Ethanol Withdrawal. J. Acupunct. Meridian Stud. 2016, 9, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Manneråsholm, L.; Shao, R.; Olsson, A.; Lönn, M.; Billig, H.; Stenervictorin, E. Electrical vs Manual Acupuncture Stimulation in a Rat Model of Polycystic Ovary Syndrome: Different Effects on Muscle and Fat Tissue Insulin Signaling. PLoS ONE 2013, 8, e54357. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Feng, Y.R.; Lonn, M.; Billig, H.; Stener, V.E. Intense electroacupuncture normalizes insulin sensitivity, increases muscle GLUT4 content, and improves lipid profile in a rat model of polycystic ovary syndrome. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E551–E559. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Waldenstrom, U.; Tagnfors, U.; Lundeberg, T.; Lindstedt, G.; Janson, P.O. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2000, 79, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Berlin, I.; Lee, T.L.; Hee, S.W.; Tan, A.S.; Picard, D.; Han, J.S. A standardized transcutaneous electric acupoint stimulation for relieving tobacco urges in dependent smokers. Evid.-Based Complement. Altern. Med. 2011, 2011, 195714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, H.F.; Fan, Y.H.; Lu, B.; Meng, L.N.; Chen, J.D. Effects and mechanisms of transcutaneous electroacupuncture on chemotherapy-induced nausea and vomiting. Evid.-Based Complement. Altern. Med. 2014, 2014, 860631. [Google Scholar] [CrossRef] [PubMed]
- Assila, B.S.; Fatma, M.; Olfa, K.; Malek, S.; Faten, H.B.A.; Sondes, H.; Faouzi, J.; Mounir, A.; Muhammad, A.E.; Mourad, A. Vascular endothelial growth factor (VEGFA) gene variation in polycystic ovary syndrome in a Tunisian women population. BMC Genom. 2016, 17, 748. [Google Scholar]
- Di Pietro, M.; Parborell, F.; Irusta, G.; Pascuali, N.; Bas, D.; Bianchi, M.S.; Tesone, M.; Abramovich, D. Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model. Endocrinology 2015, 156, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Pietro, M.D.; Scotti, L.; Irusta, G.; Tesone, M.; Parborell, F.; Abramovich, D. Local administration of platelet-derived growth factor B (PDGFB) improves follicular development and ovarian angiogenesis in a rat model of Polycystic Ovary Syndrome. Mol. Cell. Endocrinol. 2016, 433, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.M.; Wilson, H.; Rudge, J.S.; Wiegand, S.J. Single injections of vascular endothelial growth factor trap block ovulation in the macaque and produce a prolonged, dose-related suppression of ovarian function. J. Clin. Endocrinol. Metab. 2005, 90, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Wallace, J.; Kim, S.Y.; Kalyanasundaram, S.; Andalman, A.S.; Davidson, T.J.; Mirzabekov, J.J.; Zalocusky, K.A.; Mattis, J.; Denisin, A.K. Structural and molecular interrogation of intact biological systems. Nature 2013, 497, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Tomer, R.; Ye, L.; Hsueh, B.; Deisseroth, K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat. Protoc. 2014, 9, 1682–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Cui, P.; Tong, X.; Hu, W.; Shao, L.R.; Zhang, F.; Li, X.; Feng, Y. Endogenous Ovarian Angiogenesis in Polycystic Ovary Syndrome-Like Rats Induced by Low-Frequency Electro-Acupuncture: The CLARITY Three-Dimensional Approach. Int. J. Mol. Sci. 2018, 19, 3500. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113500
Ma T, Cui P, Tong X, Hu W, Shao LR, Zhang F, Li X, Feng Y. Endogenous Ovarian Angiogenesis in Polycystic Ovary Syndrome-Like Rats Induced by Low-Frequency Electro-Acupuncture: The CLARITY Three-Dimensional Approach. International Journal of Molecular Sciences. 2018; 19(11):3500. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113500
Chicago/Turabian StyleMa, Tong, Peng Cui, Xiaoyu Tong, Wei Hu, Linus R. Shao, Feifei Zhang, Xin Li, and Yi Feng. 2018. "Endogenous Ovarian Angiogenesis in Polycystic Ovary Syndrome-Like Rats Induced by Low-Frequency Electro-Acupuncture: The CLARITY Three-Dimensional Approach" International Journal of Molecular Sciences 19, no. 11: 3500. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113500