Structure–Activity Relationship Study of Newly Synthesized Iridium-III Complexes as Potential Series for Treating Thrombotic Diseases
Abstract
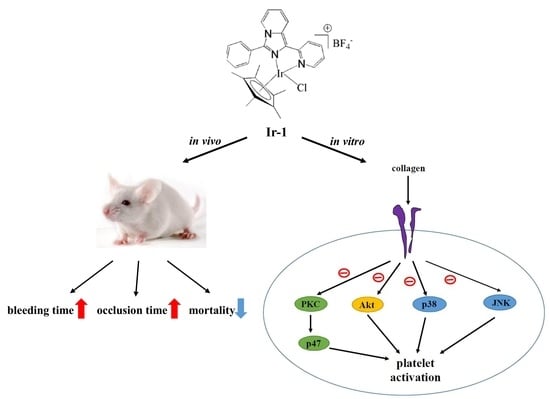
:1. Introduction
2. Results
2.1. Effects of Synthetic Ir-III Compounds on Platelet Aggregation In Vitro
2.2. Ir-III Compounds on ATP Release and [Ca2+]i Mobilization in Human Platelets
2.3. Ir-1 Attenuated Collagen Induced P-Selectin Expression without Causing Cytotoxicity
2.4. Ir-1 Tempered MAPK and Akt/PKC Phosphorylation
2.5. Ir-1 Inhibits Platelet Aggregation via Interrupting the Association of JAQ-1 with GPVI
2.6. Ir-1 Restricts Cell Adhesion and Spreading on Immobilized Collagen
2.7. Ir-1 on Closure Time
2.8. Effects of Ir Complexes on Occlusion Time and Bleeding Time
2.9. Ir-Complexes on ADP-Induced Acute Pulmonary Thromboembolism
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Synthesis of Ligands and Ir-III Complexes
4.3. Platelet Aggregation and ATP Release Assay
4.4. Measurement of Relative [Ca2+]i Mobilization
4.5. Flow Cytometric Analysis
4.6. Detection of Lactate Dehydrogenase (LDH)
4.7. Immunoblotting
4.8. Measurement of Closure Time Using PFA-100™ Platelet Function Analyzer
4.9. Confocal Microscopic Analysis of Platelet Adhesion and Spreading
4.10. Animals
4.11. Fluorescein Sodium-Induced Platelet Thrombi in Mesenteric Microvessels of Mice
4.12. Measurement of Bleeding Time in Mouse Tail Vein
4.13. Acute Pulmonary Thrombosis Induced by ADP in Mice
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, J.N.; Negrelli, J.M.; Manek, M.B.; Hawes, E.M.; Viera, A.J. Diagnosis and management of acute coronary syndrome: An evidence-based update. J. Am. Board Fam. Med. 2015, 28, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Angiolillo, D.J. Novel antiplatelet agents in acute coronary syndrome. Nat. Rev. Cardiol. 2015, 12, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, N.; Hohlfeld, T. Clinical implications of aspirin resistance. Thromb. Haemost. 2008, 100, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H. Antiplatelet resistance with aspirin and clopidogrel: Is it real and does it matter? Curr. Cardiol. Rep. 2006, 8, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Algra, A.; De Schryver, E.L.; van Gijn, J.; Kappelle, L.J.; Koudstaal, P.J. Oral anticoagulants versus antiplatelet therapy for preventing further vascular events after transient ischaemic attack or minor stroke of presumed arterial origin. Cochrane Database Syst. Rev. 2006, 3, CD001342. [Google Scholar]
- Belloc, C.; Lu, H.; Soria, C.; Fridman, R.; Legrand, Y.; Menashi, S. The effect of platelets on invasiveness and protease production of human mammary tumor cells. Int. J. Cancer 1995, 60, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Boucharaba, A.; Serre, C.M.; Gres, S.; Saulnier-Blache, J.S.; Bordet, J.C.; Guglielmi, J.; Clézardin, P.; Peyruchaud, O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Investig. 2004, 114, 1714–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, J.; Zeisig, R.; Fichtner, I. Inhibition of metastasis in a murine 4T1 breast cancer model by liposomes preventing tumor cell-platelet interactions. Clin. Exp. Metast. 2010, 27, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.H.; Nawarskas, J.J.; Frishman, W.H. Prasugrel: A new antiplatelet drug for the prevention and treatment of cardiovascular disease. Cardiol. Rev. 2008, 16, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Krötz, F.; Sohn, H.Y.; Klauss, V. Antiplatelet drugs in cardiological practice: Established strategies and new developments. Vasc. Health Risk Manag. 2008, 4, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic anticancer compounds. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, J.; Rodríguez, V.; Cutillas, N.; Samper, K.G.; Capdevila, M.; Palacios, O.; Espinosa, A. Novel C, N-chelate rhodium (III) and iridium (III) antitumor complexes incorporating a lipophilic steroidal conjugate and their interaction with DNA. Dalton Trans. 2012, 41, 12847–12856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sadler, P.J. Organoiridium complexes: Anticancer agents and catalysts. ACC Chem. Res. 2014, 47, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, C.P.; Zhang, W.; He, L.; Ji, L.N.; Mao, Z.W. Phosphorescent iridium (III)-bis-N-heterocyclic carbene complexes as mitochondria-targeted theranostic and photodynamic anticancer agents. Biomaterials 2015, 39, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.W.; Velusamy, M.; Tsao, J.T.; Hsia, C.H.; Chou, D.S.; Jayakumar, T.; Lee, L.W.; Li, J.Y.; Sheu, J.R. New Therapeutic Agent against Arterial Thrombosis: An Iridium (III)-Derived Organometallic Compound. Int. J. Mol. Sci. 2017, 18, 2616. [Google Scholar] [CrossRef] [PubMed]
- Shyu, K.G.; Velusamy, M.; Hsia, C.W.; Yang, C.H.; Hsia, C.H.; Chou, D.S.; Jayakumar, T.; Sheu, J.R.; Li, J.Y. Novel iridium (III) -derived organometallic compound for the inhibition of human platelet activation. Int. J. Mol. Med. 2018, 41, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Shyu, R.S.; Khamrang, T.; Sheu, J.R.; Hsia, C.W.; Velusamy, M.; Hsia, C.H.; Chou, D.S.; Chang, C.C. Ir-6: A Novel Iridium (III) Organometallic Derivative for Inhibition of Human Platelet Activation. Bioinorg. Chem. Appl. 2018. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.B.; Selak, M.A.; Dangelmaier, C.; Daniei, J.L. Cytosolic calcium as a second messenger for collagen-induced platelet responses. Biochem. J. 1992, 288, 925–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsig, L.; Wong, R.; Feramisco, J.; Nadeau, D.R.; Varki, N.M.; Varki, A. Heparin and cancer revisited: Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. USA 2001, 98, 3352–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, F.; Kauskot, A.; Rosa, J.P.; Bryckaert, M. Mitogen-activated protein kinases in hemostasis and thrombosis. J. Thromb. Haemost. 2008, 6, 2007–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Ostberg, O.; Wihlborg, A.K.; Brogren, H.; Jern, S.; Erlinge, D. Quantification of ADP and ATP receptor expression in human platelets. J. Thromb. Haemost. 2003, 1, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahaut-Smith, M.P. The unique contribution of ion channels to platelet and megakaryocyte function. J. Thromb. Haemost. 2012, 10, 1722–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, C.Y.; Jones, S.; Ntrakwah, A.; Naseem, K.M.; Farndale, R.W.; Mahaut-Smith, M.P. Platelet Ca2+ responses coupled to glycoprotein VI and Toll-like receptors persist in the presence of endothelial-derived inhibitors: Roles for secondary activation of P2X1 receptors and release from intracellular Ca2+ stores. Blood 2012, 119, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.J.; Schön, M.P.; Boehncke, W.H. P-selectin: A common therapeutic target for cardiovascular disorders, inflammation and tumour metastasis. Expert Opin. Ther. Targets 2007, 11, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Alghatani, M.; Heptinstall, S. Novel strategies for assessing platelet reactivity. Future Cardiol. 2017, 13, 33–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tynngard, N. Preparation, storage and quality control of platelet concentrates. Transfus. Apher. Sci. 2009, 41, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Yap, C.L.; Anderson, K.E. Phosphoinositide 3-kinases and the regulation of platelet function. Biochem. Soc. Trans. 2014, 32, 387–392. [Google Scholar] [CrossRef]
- Deb, T.B.; Coticchia, C.M.; Dickson, R.B. Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J. Biol. Chem. 2004, 279, 38903–38911. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; De, S.; Damron, D.S.; Chen, W.S.; Hay, N.; Byzova, T.V. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood 2004, 104, 1703–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazharian, A.; Roger, S.; Berrou, E.; Adam, F.; Kauskot, A.; Nurden, P.; Jandrot-Perrus, M.; Bryckaert, M. Protease-activating receptor-4 induces full platelet spreading on a fibrinogen matrix: Involvement of ERK2 and p38 and Ca2+ mobilization. J. Biol. Chem. 2007, 282, 5478–5487. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Kauskot, A.; Nurden, P.; Sulpice, E.; Hoylaerts, M.F.; Davis, R.J.; Rosa, J.P.; Bryckaert, M. Platelet JNK1 is involved in secretion and thrombus formation. Blood 2010, 115, 4083–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. Clinical utility of the PFA-100. Semin. Thromb. Hemost. 2008, 34, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.J.; Gemmell, R.; Brighton, T.A. Platelet function testing in uraemic patients. Hematology 2008, 13, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Arrieta-Blanco, J.J.; Bartolomé-Villar, B.; Juzgado, A.; Mourelle Martinez, R. Assessment of PFA-100 system for the measurement of bleeding time in oral surgery. Med. Oral Patol. Oral Cir. Bucal. 2006, 11, E514. [Google Scholar] [PubMed]
- Karger, R.; Reuter, K.; Rohlfs, J.; Nimsky, C.; Sure, U.; Kretschmer, V. The Platelet function analyzer (PFA-100) as a screening tool in neurosurgery. ISRN Hematol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Induruwa, I.; Moroi, M.; Bonna, A.; Malcor, J.D.; Howes, J.M.; Warburton, E.A.; Farndale, R.W.; Jung, S.M. Platelet collagen receptor Glycoprotein VI-dimer recognizes fibrinogen and fibrin through their D-domains, contributing to platelet adhesion and activation during thrombus formation. J. Thromb. Haemost. 2018, 16, 389–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habtemariam, A.; Melchart, M.; Ferna´ndez, R.; Parsons, S.; Oswald, I.D.H.; Parkin, A.; Fabbiani, F.P.; Davidson, J.E.; Dawson, A.; Aird, R.E.; et al. Structure-Activity Relationships for cytotoxic ruthenium(II) arene complexes containing N,N-, N,O-, and O,O-chelating ligands. J. Med. Chem. 2006, 49, 6858–6868. [Google Scholar] [CrossRef] [PubMed]
- Manso, A.; Escudero, C.; Alijo, M.; López-Fandiño, R. Platelet aggregation inhibitory activity of bovine, ovine, and caprine κ-casein macropeptides and their tryptic hydrolysates. J. Food. Prot. 2002, 65, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Alevriadou, B.R.; McIntire, L.V.; Lasslo, A. Inhibition of platelet adhesion and thrombus formation on a collagen-coated surface by novel carbamoylpiperidine antiplatelet agents. Biochim. Biophys. Acta 1992, 1137, 279–286. [Google Scholar] [CrossRef]
- Wang, J.; Dyers, L.; Mason, R.; Amoyaw, P.; Bu, X.R. Highly efficient and direct heterocyclization of dipyridyl ketone to N, N-bidentate ligands. J. Org. Chem. 2005, 70, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.A.; Huang, T.N.; Matheson, T.W.; Smith, A.K. (η6-Hexa methylbenzene) ruthenium complexes. Inorg. Synth. 1982, 21, 74–78. [Google Scholar]
- Sheu, J.R.; Lee, C.R.; Lin, C.H.; Hsiao, G.; Ko, W.C.; Chen, Y.C.; Yen, M.H. Mechanisms involved in the antiplatelet activity of Staphylococcus aureus lipoteichoic acid in human plalets. Thromb. Haemost. 2000, 83, 777–784. [Google Scholar] [PubMed]
- Jilma, B. Platelet function analyzer (PFA100): A tool to quantify congenital or acquired platelet dysfunction. J. Lab. Clin. Med. 2001, 138, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Kuo, J.R.; Lu, W.J.; Chung, C.L.; Chou, D.S.; Huang, S.Y.; Lee, H.C.; Sheu, J.R. Hinokitiol inhibits platelet activation ex vivo and thrombus formation in vivo. Biochem. Pharmacol. 2013, 85, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Lee, J.J.; Chou, D.S.; Jayakumar, T.; Fong, T.H.; Hsiao, G.; Sheu, J.R. Anovelroleofandrographolide, an NF-κB inhibitor, on inhibition of platelet activation: The pivotal mechanisms of endothelial nitric oxide synthase/cyclic GMP. J. Mol. Med. 2011, 89, 1261–1273. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-H.; Hsia, C.-W.; Jayakumar, T.; Sheu, J.-R.; Hsia, C.-H.; Khamrang, T.; Chen, Y.-J.; Manubolu, M.; Chang, Y. Structure–Activity Relationship Study of Newly Synthesized Iridium-III Complexes as Potential Series for Treating Thrombotic Diseases. Int. J. Mol. Sci. 2018, 19, 3641. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113641
Yang C-H, Hsia C-W, Jayakumar T, Sheu J-R, Hsia C-H, Khamrang T, Chen Y-J, Manubolu M, Chang Y. Structure–Activity Relationship Study of Newly Synthesized Iridium-III Complexes as Potential Series for Treating Thrombotic Diseases. International Journal of Molecular Sciences. 2018; 19(11):3641. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113641
Chicago/Turabian StyleYang, Chih-Hao, Chih-Wei Hsia, Thanasekaran Jayakumar, Joen-Rong Sheu, Chih-Hsuan Hsia, Themmila Khamrang, Yen-Jen Chen, Manjunath Manubolu, and Yi Chang. 2018. "Structure–Activity Relationship Study of Newly Synthesized Iridium-III Complexes as Potential Series for Treating Thrombotic Diseases" International Journal of Molecular Sciences 19, no. 11: 3641. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113641