Infectious Agents as Stimuli of Trained Innate Immunity
Abstract
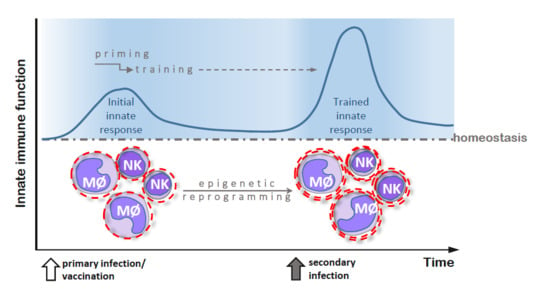
:1. Introduction
2. Trained Innate Immunity—General Characteristics and Mechanisms
3. Biochemical and Cellular Mechanisms of Training
3.1. β-Glucan
3.2. Chitin
3.3. Lipopolysaccharide (LPS)
3.4. Bacille Calmette-Guérin (BCG)
3.5. Plasmodium falciparum
3.6. Hepatitis B
4. Significance of the Trained Innate Immunity Phenomenon
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olive, A.J.; Sassetti, C.M. Metabolic crosstalk between host and pathogen: Sensing, adapting and competing. Nat. Rev. Microbiol. 2016, 14, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.A.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 8th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; ISBN 9780323222754. [Google Scholar]
- Crișan, T.O.; Netea, M.G.; Joosten, L.A. Innate immune memory: Implications for host responses to damage-associated molecular patterns. Eur. J. Immunol. 2016, 46, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, L.; Ifrim, D.C.; Moretti, S.; Tocci, N.; Cheng, S.-C.; Quintin, J.; Renga, G.; Oikonomou, V.; de Filippo, C.; Weil, T.; et al. Fungal chitin induces trained immunity in human monocytes during cross-talk of the host with Saccharomyces cerevisiae. J. Biol. Chem. 2016, 291, 7961–7972. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G. Training innate immunity: The changing concept of immunological memory in innate host defence. Eur. J. Clin. Investig. 2013, 43, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Jeffrey, K.L. Beyond receptors and signaling: Epigenetic factors in the regulation of innate immunity. Immunol. Cell Biol. 2015, 93, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, E.; Boraschi, D.; Italian, P. Innate immune memory: The latest frontier of adjuvanticity. J. Immunol. Res. 2015. [CrossRef] [PubMed]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.-J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Van Crevel, R. BCG-induced protection: Effects on innate immune memory. Semin. Immunol. 2014, 26, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Garber, M.; Yosef, N.; Goren, A.; Raychowdhury, R.; Thielke, A.; Guttman, M.; Robinson, J.; Minie, B.; Chevrier, N.; Itzhaki, Z.; et al. A high-throughput chromatin immmunoprecipitation approach reveals principles of dynamic gene regulation in mammals. Mol. Cell 2012, 47, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Nicodeme, E.; Jeffrey, K.L.; Schaefer, U.; Beinke, S.; Dewell, S.; Chung, C.W.; Chandwani, R.; Marazzi, I.; Wilson, P.; Coste, H.; et al. Suppression of inflammation by a synthetic histone mimic. Nature 2010, 468, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, R.; Piccolo, V.; Barozzi, I.; Polletti, S.; Termanini, A.; Bonifacio, S.; Curina, A.; Prosperini, E.; Ghisletti, S.; Natoli, G. Latent enhancers activated by stimulation in differentiated cells. Cell 2013, 152, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Saccani, S.; Natoli, G. Dynamic changes in histone H3 Lys 9 methylation occurring at tightly regulated inducible inflammatory genes. Genes Dev. 2002, 16, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.C.; Schaefer, U.; Mecklenbrauker, I.; Stienen, A.; Dewell, S.; Chen, M.S.; Rioja, I.; Parravicini, V.; Prinjha, R.K.; Chandwani, R. Histone H3 lysine 9 di-methylation as an epigenetic signature of the interferon response. J. Exp. Med. 2012, 209, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Maia, B.M.; Rocha, R.M.; Calin, G.A. Clinical significance and the interaction between non-coding RNAs and the epigenetics machinery challenges and opportunities in oncology. Epigenetics 2014, 9, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin. Epigenet. 2015, 7, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, S.; Asadzadeh-Aghdaei, H.; Nazemalhosseini-Mojarad, E.; Dabiri, H.; Ghanbari, R.; Reza Zali, M. Gut microbiota, epigenetic modification and colorectal cancer. Iran. J. Microbiol. 2017, 9, 55–63. [Google Scholar] [PubMed]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C-W.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C-S. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011, 478, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hard, T. Role of microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Frellstedt, L.; Waldschmidt, I.; Gosset, P.; Desmet, C.; Pirottin, D.; Bureau, F.; Farnir, F.; Franck, T.; Dupuis-Tricaud, M.-C.; Lekeux, P.; et al. Training modifies innate immune responses in blood monocytes and in pulmonary alveolar macrophages. Am. J. Respir. Cell Mol. 2014, 51, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.A.; D’Enfert, C.; Bougnoux, M.E. Candida albicans commensalism in the gastrointestinal tract. FEMS Yeast Res. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.; Correia, I.; Pla, J.; Román, E. Adaptation of Candida albicans to commensalism in the gut. Future Microbiol. 2016, 11, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Ifrim, D.C.; Quintin, J.; Meerstein-Kessel, L.; Plantinga, T.S.; Joosten, L.A.; Van der Meer, J.W.; Van de Veerdonk, F.L.; Netea, M.G. Defective trained immunity in patients with STAT-1-dependent chronic mucocutaneaous candidiasis. Clin. Exp. Immunol. 2015, 181, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Buro, L.J.; Chipumuro, E.; Henriksen, M.A. Menin and RNF20 recruitment is associated with dynamic histone modifications that regulate signal transducer and activator of transcription 1 (STAT1)-activated transcription of the interferon regulatory factor 1 gene (IRF1). Epigenet. Chromatin 2010, 3. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Valtanen, P.; Guzman-Genuino, R.M.; Williams, D.L.; Hayball, J.D.; Diener, K.R. Evaluation of trained immunity by β-1, 3 (d)-glucan on murine monocytes in vitro and duration of response in vivo. Immunol. Cell Biol. 2017, 95, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Netea, M.G. Treatment options for chronic mucocutaneous candidiasis. J. Infect. 2016, 72, S56–S60. [Google Scholar] [CrossRef] [PubMed]
- Shrive, A.K.; Moeller, J.B.; Burns, I.; Paterson, J.M.; Shaw, A.J.; Schlosser, A.; Sorensen, G.L.; Greenhough, T.J.; Holmskov, U. Crystal structure of the tetrameric fibrinogen-like recognition domain of fibrinogen C domain containing 1 (FIBCD1) protein. J. Biol. Chem. 2014, 289, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Trent, M.S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.M.; Mills, K.H.G. The cells that mediate innate immune memory and their functional significance in inflammatory and infectious diseases. Semin. Immunol. 2016, 28, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Bistoni, F.; Vecchiarelli, A.; Cenci, E.; Puccetti, P.; Marconi, P.; Cassone, A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 1986, 51, 668–674. [Google Scholar] [PubMed]
- Bistoni, F.; Verducci, G.; Perito, S.; Vecchiarelli, A.; Puccetti, P.; Marconi, P.; Cassone, A. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J. Med. Vet. Mycol. 1988, 26, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Van’t Wout, J.W.; Poell, R.; van Furth, R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 1992, 36, 713–719. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Cheng, S.C.; van der Meer, J.W.M.; Netea, M.G. Innate immune memory: Towards a better understanding of host defense mechanism. Curr. Opin. Immunol. 2014, 29. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; van Crevel, R.; Netea, M.G. Trained immunity: Consequences for the heterologous effects of BCG vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lerm, M.; Netea, M.G. Trained immunity: A new avenue for tuberculosis vaccine development. J. Intern. Med. 2016, 279, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Blok, B.A.; Arts, R.J.W.; van Crevel, R.; Benn, C.S.; Netea, M.G. Trained innate immunity as underlaying mechanism for the long-term, nonspecific effects of vaccines. J. Leukoc. Biol. 2015, 98, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.B.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In-vitro experimental model of trained innate immunity in human primary monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Brayner, F.A.; Alves, L.C.; Dixit, R.; Barillas-Mury, C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 2010, 329, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.B.B.; Netea, M.G.; Hermsen, C.C.; Jansen, T.; Jacobs, L.; Golenbock, D.; van der Ven, A.J.A.M.; Sauerwein, R.W. Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J. Immunol. 2007, 179, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Luty, A.J.F.; Lell, B.; Schmidt-Ott, R.; Lehman, L.G.; Luckner, D.; Greve, B.; Matousek, P.; Herbich, K.; Schmid, D.; Migot-Nabias, F.; et al. Interferon-γ responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J. Infect. Dis. 1999, 179, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, D.; Omer, F.M.; Todd, J.; Akanmori, B.D.; Koram, K.A.; Riley, E.M. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J. Infect. Dis. 2002, 185, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Bertoletti, A. Tolerance and immunity to pathogens in early life: Insights from HBV infection. Semin. Immunopathol. 2017. [CrossRef] [PubMed]
- Hong, M.; Sandalova, E.; Low, D.; Gehring, A.J.; Fieni, S.; Amadei, B.; Urbani, S.; Chong, Y.-S.; Guccione, E.; Bertoletti, A. Trained immunity in newborn infants of HBV-infected mothers. Nat. Commun. 2014, 6. [Google Scholar] [CrossRef]
- Levy, O.; Wynn, J.L. A prime time for trained immunity: Innate immune memory in newborns and infants. Neonatology 2014, 105, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Strunk, T.; Prosser, A.; Levy, O.; Philbin, V.; Simmer, K.; Doherty, D.; Charles, A.; Richmond, P.; Burgner, D.; Currie, A. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediatr. Res. 2012, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kronforst, K.D.; Mancuso, C.J.; Pettengill, M.; Ninkovic, J.; Coombs, M.R.P.; Stevens, C.; Otto, M.; Mallard, C.; Wang, X.; Goldmann, D.; et al. A neonatal model of intravenous Staphylococcus epidermidis infection in mice <24 h old enables characterization of early innate immune responses. PLoS ONE 2012, 7, e43897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaby, P.; Samb, B.; Simondon, F.; Seck, A.M.; Knudsen, K.; Whittle, H. Non-specific beneficial effect of measles immunisation: Analysis of mortality studies from developing countries. Br. Med. J. 1995, 311, 481–485. [Google Scholar] [CrossRef]
- Pfahlberg, A.; Kölmel, K.F.; Grange, J.M.; Mastrangelo, G.; Krone, B.; Botev, I.N.; Niin, M.; Seebacher, C.; Lambert, D.; Shafir, R.; et al. Inverse association between melanoma and previous vaccinations against tuberculosis and smallpox: Results of the FEBIM study. J. Investig. Dermatol. 2002, 119, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Garly, M.L.; Jensen, H.; Nielsen, J.; Aaby, P. Bacillus Calmette-Guérin vaccination and infant mortality. Expert Rev. Vaccines 2006, 5, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Sørup, S.; Benn, C.S.; Poulsen, A.; Krause, T.G.; Aaby, P.; Ravn, H. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA 2014, 311, 826–835. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.J.; Pardo-Seco, J.; Martinón-Torres, F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin. Infect. Dis. 2015, 60, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Benn, C.; Nielsen, J.; Lisse, I.M.; Rodrigues, A.; Ravn, H. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Benn, C.S.; van Crevel, R. Unravelling the nature of non-specific effects of vaccines—A challenge for innate immunologists. Semin. Immunol. 2016, 28, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.B.C.; Netea, M.G.; Kater, A.P.; van der Velden, W.J.F.M. “Trained immunity”: Consequences for lymphoid malignancies. Haematologica 2016, 101, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, A.; Hassanzadeh-Kiabi, N.; Ng, M.Y.; Megías, J.; Subramanian, A.; Liu, G.Y.; Underhill, D.M.; Gil, M.L.; Goodridge, H.S. Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur. J. Immunol. 2013, 43, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Askenase, M.H.; Han, S.J.; Byrd, A.L.; Morais da Fonseca, D.; Bouladoux, N.; Wilhelm, C.; Konkel, J.E.; Hand, T.W.; Lacerda-Queiroz, N.; Su, X.Z.; et al. Bone-marrow-resident NK cells prime monocytes for regulatory function during infection. Immunity 2015, 42, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.L.; Buonomo, E.; Carey, M.; Cowardin, C.; Naylor, C.; Noor, Z.; Wills-Karp, M.; Petri, W.A., Jr. Bone marrow dendritic cells from mice with an altered microbiota provide interleukin 17A-dependent protection against Entamoeba histolytica colitis. MBio 2014, 5, e01817. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusek, P.; Wala, M.; Druszczyńska, M.; Fol, M. Infectious Agents as Stimuli of Trained Innate Immunity. Int. J. Mol. Sci. 2018, 19, 456. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020456
Rusek P, Wala M, Druszczyńska M, Fol M. Infectious Agents as Stimuli of Trained Innate Immunity. International Journal of Molecular Sciences. 2018; 19(2):456. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020456
Chicago/Turabian StyleRusek, Paulina, Mateusz Wala, Magdalena Druszczyńska, and Marek Fol. 2018. "Infectious Agents as Stimuli of Trained Innate Immunity" International Journal of Molecular Sciences 19, no. 2: 456. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020456