The Influence of Selective Laser Melting (SLM) Process Parameters on In-Vitro Cell Response
Abstract
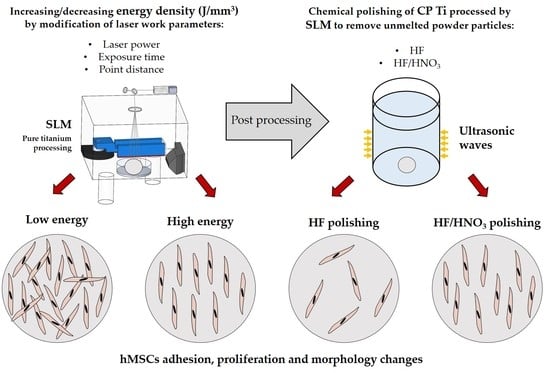
:1. Introduction
2. Results
2.1. Influence of the SLM Process Parameters on the Properties of CP Titanium
2.2. Influence of Chemical and Heat Treatments on the Properties of CP Titanium
2.3. Surface Chemistry
2.4. Residual Stresses
2.5. Cell Behavior
3. Discussion
3.1. Process Conditions
3.2. Surface Chemistry and Residual Stresses
3.3. Cell Behavior
4. Materials and Methods
4.1. Fabrication of Samples
4.2. Chemical and Heat Treatments
4.3. Contact Angle and Surface Energy
4.4. X-ray Photoelectron Spectroscopy (XPS)
4.5. Determination of Residual Stresses
4.6. Cell Culture
4.7. Cell Observations
5. Conclusions
6. Patents
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Laser Power lp (W) | Exposure Time exp (µs) | Point Distance pd (µm) | Scanning Speed ν (mm/s) | Energy Density (J/mm3) |
|---|---|---|---|---|
| Increasing laser power (lp) | ||||
| 25 | 40 | 20 | 500 | 12.50 |
| 35 | 40 | 20 | 500 | 17.50 |
| 45 | 40 | 20 | 500 | 22.50 |
| Increasing exposure time (exp) | ||||
| 45 | 20 | 20 | 1000 | 11.25 |
| 45 | 40 | 20 | 500 | 22.50 |
| 45 | 60 | 20 | 333 | 33.75 |
| 45 | 80 | 20 | 250 | 45.00 |
| Increasing point distance (pd) | ||||
| 45 | 40 | 20 | 500 | 22.50 |
| 45 | 40 | 40 | 1000 | 11.25 |
| 45 | 40 | 60 | 1500 | 7.50 |
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Contact Angle (°) | σ (°) | Surface Energy (mN/m) | Dispersive Energy (mN/m) | Polar Energy (mN/m) | ch2/n (mN/m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF-HNO3|water | 119 | 117 | 115 | 117 | 119 | 115 | 117 | 114 | 116 | 118 | 120 | 115 | 114 | 114 | 118 | 116 | 2 | 152 | 123 | 28 | 4.36 × 10−15 |
| HF-HNO3|formamide | 87 | 88 | 88 | 89 | 86 | 89 | 87 | 83 | 86 | 83 | 87 | 85 | 87 | 90 | 91 | 87 | 2 | ||||
| HF|water | 130 | 127 | 127 | 129 | 129 | 126 | 127 | 129 | 131 | 126 | 129 | 125 | 126 | 128 | 130 | 128 | 2 | 81 | 65 | 16 | 5.88 × 10−15 |
| HF|formamide | 107 | 103 | 107 | 104 | 104 | 103 | 106 | 105 | 107 | 104 | 105 | 104 | 104 | 104 | 105 | 105 | 1 | ||||
| 600 C (oxygen)|water | 76 | 70 | 75 | 73 | 69 | 68 | 67 | 76 | 74 | 72 | 73 | 74 | 73 | 76 | 70 | 72 | 3 | 55 | 54 | 1 | 8.60 × 10−15 |
| 600 C (oxygen)|formamide | 70 | 80 | 90 | 89 | 84 | 79 | 90 | 92 | 67 | 81 | 62 | 61 | 63 | 92 | 74 | 78 | 11 | ||||
| 600 C (vacuum)|water | 89 | 88 | 91 | 87 | 87 | 89 | 90 | 91 | 91 | 91 | 94 | 90 | 88 | 93 | 88 | 90 | 2 | 75 | 4 | 71 | 4.17 × 101 |
| 600 C (vacuum)|formamide | 74 | 72 | 71 | 68 | 69 | 69 | 71 | 71 | 74 | 73 | 70 | 68 | 68 | 74 | 68 | 71 | 2 | ||||
| 25 W|water | 111 | 113 | 115 | 113 | 117 | 111 | 113 | 112 | 116 | 114 | 116 | 116 | 112 | 114 | 114 | 114 | 2 | 239 | 189 | 50 | 1.21 × 10−14 |
| 25 W|formamide | 77 | 79 | 80 | 79 | 80 | 78 | 78 | 80 | 78 | 76 | 78 | 78 | 75 | 73 | 78 | 78 | 2 | ||||
| 35 W|water | 121 | 120 | 123 | 120 | 122 | 121 | 123 | 126 | 123 | 124 | 126 | 124 | 126 | 123 | 124 | 123 | 2 | 273 | 205 | 68 | 2.43 × 10−14 |
| 35 W|formamide | 86 | 84 | 83 | 80 | 81 | 84 | 87 | 86 | 88 | 82 | 88 | 87 | 85 | 84 | 81 | 85 | 3 | ||||
| 45 W (As made)|water | 109 | 107 | 108 | 110 | 110 | 109 | 107 | 107 | 110 | 109 | 109 | 110 | 107 | 111 | 108 | 109 | 1 | 294 | 234 | 61 | 1.42 × 10−14 |
| 45 W (As made)|formamide | 71 | 67 | 66 | 69 | 68 | 69 | 71 | 67 | 69 | 70 | 68 | 73 | 72 | 65 | 69 | 69 | 2 | ||||
| exp20|water | 113 | 117 | 120 | 115 | 112 | 114 | 120 | 121 | 121 | 117 | 115 | 119 | 120 | 121 | 113 | 117 | 3 | 193 | 153 | 40 | 5.63 × 10−15 |
| exp20|formamide | 83 | 86 | 84 | 89 | 83 | 83 | 90 | 85 | 81 | 85 | 89 | 82 | 82 | 86 | 80 | 84 | 3 | ||||
| exp40|water | 109 | 107 | 108 | 110 | 110 | 109 | 107 | 107 | 110 | 109 | 109 | 110 | 107 | 111 | 108 | 109 | 1 | 294 | 234 | 61 | 1.42 × 10−14 |
| exp40|formamide | 71 | 67 | 66 | 69 | 68 | 69 | 71 | 67 | 69 | 70 | 68 | 73 | 72 | 65 | 69 | 69 | 2 | ||||
| exp60|water | 98 | 97 | 96 | 98 | 97 | 99 | 98 | 99 | 99 | 100 | 98 | 100 | 97 | 98 | 98 | 98 | 1 | 267 | 224 | 43 | 8.44 × 10−15 |
| exp60|formamide | 58 | 57 | 58 | 57 | 59 | 57 | 60 | 60 | 58 | 58 | 58 | 58 | 59 | 59 | 61 | 58 | 1 | ||||
| exp80|water | 105 | 102 | 106 | 105 | 105 | 104 | 104 | 103 | 105 | 108 | 102 | 102 | 104 | 103 | 108 | 104 | 2 | 466 | 362 | 104 | 1.22 × 10−14 |
| exp80|formamide | 50 | 55 | 54 | 54 | 50 | 55 | 51 | 55 | 55 | 50 | 53 | 55 | 52 | 51 | 53 | 53 | 2 | ||||
| pd20|water | 109 | 107 | 108 | 110 | 110 | 109 | 107 | 107 | 110 | 109 | 109 | 110 | 107 | 111 | 108 | 109 | 1 | 294 | 234 | 61 | 1.42 × 10−14 |
| pd20|formamide | 71 | 67 | 66 | 69 | 68 | 69 | 71 | 67 | 69 | 70 | 68 | 73 | 72 | 65 | 69 | 69 | 2 | ||||
| pd40|water | 126 | 128 | 129 | 132 | 131 | 127 | 132 | 126 | 127 | 131 | 127 | 130 | 126 | 128 | 127 | 128 | 2 | 159 | 122 | 38 | 1.29 × 10−14 |
| pd40|formamide | 100 | 99 | 102 | 101 | 100 | 97 | 99 | 96 | 96 | 99 | 103 | 95 | 98 | 97 | 98 | 99 | 2 | ||||
| pd60|water | 126 | 129 | 126 | 126 | 128 | 129 | 130 | 127 | 128 | 127 | 127 | 126 | 130 | 130 | 126 | 128 | 1 | 145 | 111 | 34 | 9.19 × 10−15 |
| pd60|formamide | 99 | 94 | 94 | 95 | 97 | 97 | 98 | 97 | 96 | 97 | 99 | 98 | 96 | 97 | 96 | 97 | 1 |
Appendix B

References
- Chen, X.; Possel, J.K.; Wacongne, C.; van Ham, A.F.; Klink, P.C.; Roelfsema, P.R. 3D printing and modelling of customized implants and surgical guides for non-human primates. J. Neurosci. Methods 2017, 286, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Frisardi, G.; Chessa, G.; Barone, S.; Paoli, A.; Razionale, A.; Frisardi, F. Integration of 3D anatomical data obtained by CT imaging and 3D optical scanning for computer aided implant surgery. BMC Med. Imaging 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Design for additive bio-manufacturing: From patient-specific medical devices to rationally designed meta-biomaterials. Int. J. Mol. Sci. 2017, 18, 1607. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar] [CrossRef]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Yan, C. Stiffness and strength tailoring of cobalt chromium graded cellular structures for stress-shielding reduction. Mater. Des. 2017, 114, 633–641. [Google Scholar] [CrossRef]
- Tunchel, S.; Blay, A.; Kolerman, R.; Mijiritsky, E.; Shibli, J.A. 3D Printing/Additive Manufacturing Single Titanium Dental Implants: A Prospective Multicenter Study with 3 Years of Follow-Up. Int. J. Dent. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.; Chen, X.; Zhang, C.; Zhang, G.; Xu, Z. Design and manufacture of customized dental implants by using reverse engineering and selective laser melting technology. J. Prosthet. Dent. 2018, 112, 1088–1095.e1. [Google Scholar] [CrossRef] [PubMed]
- Jamshidinia, M.; Wang, L.; Tong, W.; Ajlouni, R.; Kovacevic, R. Fatigue properties of a dental implant produced by electron beam melting® (EBM). J. Mater. Process. Technol. 2015, 226, 255–263. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Zhou, Q.; Gong, Y.; Li, R.; Li, C.; Klämpfl, F.; Freund, S.; Wu, X.; Sun, Y.; et al. Laser beam melting 3D printing of Ti6Al4V based porous structured dental implants: Fabrication, biocompatibility analysis and photoelastic study. Sci. Rep. 2017, 7, 45360. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann. Maxillofac. Surg. 2014, 4, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, N. Clinical Application of Three-Dimensional Printing Technology in Craniofacial Plastic Surgery. Arch. Plast. Surg. 2015, 42, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Mounir, M.; Atef, M.; Abou-Elfetouh, A.; Hakam, M.M. Titanium and polyether ether ketone (PEEK) patient-specific sub-periosteal implants: Two novel approaches for rehabilitation of the severely atrophic anterior maxillary ridge. Int. J. Oral Maxillofac. Surg. 2018, 47, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Sajad, A.; Burnett, J.; Michael, T.; Damiano, P. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J. Orthop. Res. 2016, 35, 1774–1783. [Google Scholar] [CrossRef]
- Goriainov, V.; McEwan, J.K.; Oreffo, R.O.C.; Dunlop, D.G. Application of 3D-printed patient-specific skeletal implants augmented with autologous skeletal stem cells. Regen. Med. 2018, 13, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Rafi, K.; Gu, H.; Starr, T.; Stucker, B. Analysis of defect generation in Ti-6Al-4V parts made using powder bed fusion additive manufacturing processes. Addit. Manuf. 2014, 1, 87–98. [Google Scholar] [CrossRef]
- Weißmann, V.; Drescher, P.; Bader, R.; Seitz, H.; Hansmann, H.; Laufer, N. Comparison of Single Ti6Al4V Struts Made Using Selective Laser Melting and Electron Beam Melting Subject to Part Orientation. Metals 2017, 7, 91. [Google Scholar] [CrossRef]
- Gong, H.; Rafi, K.; Gu, H.; Ram, G.D.J.; Starr, T.; Stucker, B. Influence of defects on mechanical properties of Ti–6Al–4V components produced by selective laser melting and electron beam melting. Mater. Des. 2015, 86, 545–554. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; Janbaz, S.; Leeflang, S.M.A.; Lietaert, K.; Weinans, H.H.; Zadpoor, A.A. Rationally designed meta-implants: A combination of auxetic and conventional meta-biomaterials. Mater. Horiz. 2018, 5, 28–35. [Google Scholar] [CrossRef]
- Tan, X.P.; Tan, Y.J.; Chow, C.S.L.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Gokuldoss, P.K.; Kolla, S.; Eckert, J. Additive Manufacturing Processes: Selective Laser Melting, Electron Beam Melting and Binder Jetting—Selection Guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Chen, H. Selective laser melting of high strength and toughness stainless steel parts: The roles of laser hatch style and part placement strategy. Mater. Sci. Eng. A 2018, 725, 419–427. [Google Scholar] [CrossRef]
- Sufiiarov, V.S.; Popovich, A.A.; Borisov, E.V.; Polozov, I.A.; Masaylo, D.V.; Orlov, A.V. The effect of layer thickness at selective laser melting. Procedia Eng. 2017, 174, 126–134. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Mian, A.; Srinivasan, R. Effect of process parameters on hardness, temperature profile and solidification of different layers processed by direct metal laser sintering (DMLS). AIP Conf. Proc. 2016, 1754, 50046. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Y.; Wang, D. Parametric optimization of selective laser melting for forming Ti6Al4V samples by Taguchi method. Opt. Laser Technol. 2013, 49, 118–124. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chuang, C.-S.; Lin, C.-C.; Wu, C.-H.; Lin, D.-Y.; Liu, S.-H.; Tseng, W.-P.; Horng, J.-B. Microstructure-controllable Laser Additive Manufacturing Process for Metal Products. Phys. Procedia 2014, 56, 58–63. [Google Scholar] [CrossRef]
- Zeng, K. Optimization of Support Structures for Selective Laser Melting. Ph.D. Thesis, University of Louisville, Louisville, KY, USA, 2015. [Google Scholar]
- Simchi, A. The role of particle size on the laser sintering of iron powder. Metall. Mater. Trans. B 2004, 35, 937–948. [Google Scholar] [CrossRef]
- Attar, H.; Prashanth, K.G.; Zhang, L.-C.; Calin, M.; Okulov, I.V.; Scudino, S.; Yang, C.; Eckert, J. Effect of Powder Particle Shape on the Properties of In Situ Ti–TiB Composite Materials Produced by Selective Laser Melting. J. Mater. Sci. Technol. 2015, 31, 1001–1005. [Google Scholar] [CrossRef]
- Vandenbroucke, B.; Kruth, J.J.-P.J. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Attar, H.; Calin, M.; Zhang, L.C.C.; Scudino, S.; Eckert, J. Manufacture by selective laser melting and mechanical behavior of commercially pure titanium. Mater. Sci. Eng. A 2014, 593, 170–177. [Google Scholar] [CrossRef]
- Wysocki, B.; Swieszkowski, W.; Kurzydlowski, K.J. Porous Titanium Scaffold Fabrication for Bone Tissue Engineering by SLM (Selective Laser Melting) Method. In Proceedings of the DDMC, Berlin, Germany, 12–13 March 2014; pp. 2–5. [Google Scholar]
- Thijs, L.; Sistiaga, M.L.M.; Wauthle, R.; Xie, Q.; Kruth, J.-P.; van Humbeeck, J. Strong morphological and crystallographic texture and resulting yield strength anisotropy in selective laser melted tantalum. Acta Mater. 2013, 61, 4657–4668. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Sitek, R.; Buhagiar, J.; Kurzydłowski, K.J.K.; Świeszkowski, W.; Święszkowski, W. Laser and Electron Beam Additive Manufacturing Methods of Fabricating Titanium Bone Implants. Appl. Sci. 2017, 7, 657. [Google Scholar] [CrossRef]
- Shishkovskii, I.V.; Yadroitsev, I.A.; Smurov, I.Y. Selective laser sintering/melting of nitinol--hydroxyapatite composite for medical applications. Powder Metall. Met. Ceram. 2011, 50, 275–283. [Google Scholar] [CrossRef]
- Yavari, S.A.; Chai, Y.C.; Böttger, A.J.; Wauthle, R.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Effects of anodizing parameters and heat treatment on nanotopographical features, bioactivity, and cell culture response of additively manufactured porous titanium. Mater. Sci. Eng. C 2015, 51, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysocki, B.; Idaszek, J.; Buhagiar, J.; Szlązak, K.; Brynk, T.; Kurzydłowski, K.J.; Święszkowski, W. The influence of chemical polishing of titanium scaffolds on their mechanical strength and in-vitro cell response. Mater. Sci. Eng. C 2018. [Google Scholar] [CrossRef]
- Cacace, S.; Demir, A.G.; Semeraro, Q. Densification mechanism for different types of stainless steel powders in selective laser melting. Procedia CIRP 2017, 62, 475–480. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W. Densification mechanism and microstructural evolution in selective laser sintering of Al–12Si powders. J. Mater. Process. Technol. 2011, 211, 113–121. [Google Scholar] [CrossRef]
- Puebla, K.; Murr, L.E.; Gaytan, S.M.; Martinez, E.; Medina, F.; Wicker, R.B. Effect of Melt Scan Rate on Microstructure and Macrostructure for Electron Beam Melting of Ti-6Al-4V. Mater. Sci. Appl. 2012, 3, 259–264. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Wu, X.; Dargusch, M.S. Comparative study of commercially pure titanium produced by laser engineered net shaping, selective laser melting and casting processes. Mater. Sci. Eng. A 2017, 705, 385–393. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Hedayati, R.; Jain, R.K.A.K.; Li, Y.; Leeflang, S.; Zadpoor, A.A. Effects of laser processing parameters on the mechanical properties, topology, and microstructure of additively manufactured porous metallic biomaterials: A vector-based approach. Mater. Des. 2017, 134, 234–243. [Google Scholar] [CrossRef]
- Schwartz, Z.; Raz, P.; Zhao, G.; Barak, Y.; Tauber, M.; Yao, H.; Boyan, B.D. Effect of Micrometer-Scale Roughness of the Surface of Ti6Al4V Pedicle Screws in Vitro and in Vivo. J. Bone Jt. Surg. Am. 2008, 90, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Gianluca, G.; Fini, M.; Chiesa, R.; Giordano, C.; Sandrini, E.; Bianchi, A.E.; Ceribelli, P.; Giardino, R. A novel multiphase anodic spark deposition coating for the improvement of orthopedic implant osseointegration: An experimental study in cortical bone of sheep. J. Biomed. Mater. Res. Part A 2007, 85A, 1022–1031. [Google Scholar] [CrossRef]
- Hallgren, C.; Reimers, H.; Chakarov, D.; Gold, J.; Wennerberg, A. An in vivo study of bone response to implants topographically modified by laser micromachining. Biomaterials 2003, 24, 701–710. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Lim, Y.J.; Oshida, Y.; Andres, C.J.; Barco, M.T. Surface characterizations of variously treated titanium materials. Int. J. Oral Maxillofac. Surg. 2001, 16, 333–342. [Google Scholar]
- Giljean, S.; Bigerelle, M.; Anselme, K.; Haidara, H. New insights on contact angle/roughness dependence on high surface energy materials. Appl. Surf. Sci. 2011, 257, 9631–9638. [Google Scholar] [CrossRef]
- Wysocki, B.; Idaszek, J.; Szlązak, K.; Strzelczyk, K.; Brynk, T.; Kurzydłowski, K.J.; Święszkowski, W. Post processing and biological evaluation of the titanium scaffolds for bone tissue engineering. Materials 2016, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Hierro-Oliva, M.; Gallardo-Moreno, A.M.; Rodríguez-Cano, A.; Bruque, J.M.; González-Martín, M.L. Experimental approach towards the water contact angle value on the biomaterial alloy Ti6Al4V. Ann. UMCS Chem. 2015, 70, 1–13. [Google Scholar] [CrossRef]
- Strnad, G.; Chirila, N.; Petrovan, C.; Russu, O. Contact Angle Measurement on Medical Implant Titanium Based Biomaterials. Procedia Technol. 2016, 22, 946–953. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Kalin, M.; Polajnar, M. The correlation between the surface energy, the contact angle and the spreading parameter, and their relevance for the wetting behaviour of DLC with lubricating oils. Tribol. Int. 2013, 66, 225–233. [Google Scholar] [CrossRef]
- Oshida, Y.; Sachdeva, R.; Miyazaki, S.; Daly, J. Effects of shot-peening on surface contact angles of biomaterials. J. Mater. Sci. Mater. Med. 1993, 4, 443–447. [Google Scholar] [CrossRef]
- Oshida, Y.; Sachdeva, R.; Miyazaki, S. Changes in contact angles as a function of time on some pre-oxidized biomaterials. J. Mater. Sci. Mater. Med. 1992, 3, 306–312. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.-P.P.; van Humbeeck, J. Heat treatment of Ti6Al4V produced by Selective Laser Melting: Microstructure and mechanical properties. J. Alloys Compd. 2012, 541, 177–185. [Google Scholar] [CrossRef]
- Tendulkar, G.; Sreekumar, V.; Rupp, F.; Teotia, A.K.; Athanasopulu, K.; Kemkemer, R.; Buck, A.; Buck, A.; Kaps, H.-P.; Geis-Gerstorfer, J.; et al. Characterisation of porous knitted titanium for replacement of intervertebral disc nucleus pulposus. Sci. Rep. 2017, 7, 16611. [Google Scholar] [CrossRef] [PubMed]
- Stevens, N.; Priest, C.I.; Sedev, R.; Ralston, J. Wettability of photoresponsive titanium dioxide surfaces. Langmuir 2003, 19, 3272–3275. [Google Scholar] [CrossRef]
- Sun, B.; Li, S.; Imai, H.; Mimoto, T.; Umeda, J.; Kondoh, K. Fabrication of high-strength Ti materials by in-process solid solution strengthening of oxygen via P/M methods. Mater. Sci. Eng. A 2013, 563, 95–100. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Święszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Murr, L.E.L.E.; Gaytan, S.M.S.M.; Ramirez, D.A.D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal Fabrication by Additive Manufacturing Using Laser and Electron Beam Melting Technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Vaithilingam, J.; Prina, E.; Goodridge, R.D.; Hague, R.J.M.; Edmondson, S.; Rose, F.R.A.J.; Christie, S.D.R. Surface chemistry of Ti6Al4V components fabricated using selective laser melting for biomedical applications. Mater. Sci. Eng. C 2016, 67, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y.F. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Khang, D.; Kim, S.Y.; Liu-Snyder, P.; Palmore, G.T.R.; Durbin, S.M.; Webster, T.J. Enhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: Independent role of surface nano-roughness and associated surface energy. Biomaterials 2007, 28, 4756–4768. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef] [PubMed]









| Parameter | Abbreviation | Value |
|---|---|---|
| Laser current (mA) | lc | 1000–1800 |
| Laser power (W) | lp | 25–45 |
| Exposure time (µs) | exp | 20–80 |
| Point distance (µm) | pd | 20–60 |
| Hatch spacing (µm) | h | 80 |
| Scanning speed (mm/s) | υ | 250 a–1500 b |
| Layer thickness (µm) | t | 50 |
| Energy density (J/mm3) | E | 8 b–45 a |
| Model size (mm) | S | φ = 12, h = 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocki, B.; Idaszek, J.; Zdunek, J.; Rożniatowski, K.; Pisarek, M.; Yamamoto, A.; Święszkowski, W. The Influence of Selective Laser Melting (SLM) Process Parameters on In-Vitro Cell Response. Int. J. Mol. Sci. 2018, 19, 1619. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061619
Wysocki B, Idaszek J, Zdunek J, Rożniatowski K, Pisarek M, Yamamoto A, Święszkowski W. The Influence of Selective Laser Melting (SLM) Process Parameters on In-Vitro Cell Response. International Journal of Molecular Sciences. 2018; 19(6):1619. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061619
Chicago/Turabian StyleWysocki, Bartłomiej, Joanna Idaszek, Joanna Zdunek, Krzysztof Rożniatowski, Marcin Pisarek, Akiko Yamamoto, and Wojciech Święszkowski. 2018. "The Influence of Selective Laser Melting (SLM) Process Parameters on In-Vitro Cell Response" International Journal of Molecular Sciences 19, no. 6: 1619. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061619