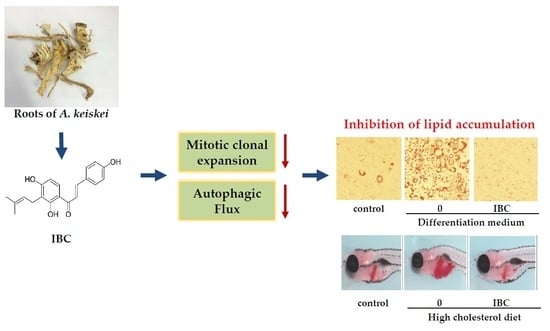
Isobavachalcone from Angelica keiskei Inhibits Adipogenesis and Prevents Lipid Accumulation
Abstract
:1. Introduction
2. Results
2.1. Isobavachalcone (IBC) Inhibits Adipocyte Proliferation and Differentiation
2.2. IBC Inhibits Mitotic Clonal Expansion via Cell Cycle Arrest
2.3. IBC Reduces Gene Expression of Transcription Factors during Adipocyte Differentiation
2.4. IBC Decreases Gene Expression of Lipid Metabolism-Regulating Factors during Adipocyte Differentiation
2.5. IBC Inhibits Autophagic Flux during Adipocyte Differentiation
2.6. IBC Inhibits Lipid Accumulation in High Cholesterol-Diet Zebrafish Larvae
3. Discussion
4. Materials and Methods
4.1. Isolation of Isobavachalcone (IBC) from Root of Angelica keiskei
4.2. Culture and Preadipocyte Differentiation
4.3. MTT Assay and Oil Red O (ORO) Staining
4.4. Flow Cytometry
4.5. Transfection and Confocal Microscopy
4.6. Zebrafish Experiments
4.7. Whole Mount ORO Staining and NBD Cholesterol Staining
4.8. RNA Extraction and Quantitative Real Time-PCR (qRCR)
4.9. Western Blot Analysis
4.10. Statistical Analysis
Author Contributions
Acknowledgements
Conflicts of Interest
References
- OECD. Available online: http://www.oecd.org/health/obesity-update.htm (accessed on 6 June 2017).
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural products with anti-obesity effects and different mechanisms of action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, P.R.L.; de Albuquerque Siebra, A.L.; de Lacerda Neto, L.J.; de Oliveira, L.R. Obesity and Natural Products. J. Food Nutr. Res. 2015, 3, 540–549. [Google Scholar]
- Hu, Y.; Davies, G.E. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia 2010, 81, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Iizuka, M.; Schneider, S.; Ogasawara, K.; Mukainaka, T.; Iwatsuki, K.; Suzuki, T.; Nishino, H. Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett. 2003, 201, 133–137. [Google Scholar] [CrossRef]
- Park, C.K.; Lee, Y.; Chang, E.J.; Lee, M.H.; Yoon, J.H.; Ryu, J.H.; Kim, H.H. Bavachalcone inhibits osteoclast differentiation through suppression of NFATc1 induction by RANKL. Biochem. Pharmacol. 2008, 75, 2175–21812. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic activities of chalcones isolated from a Japanese Herb, Angelica keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Nam, S.; Li, H.; Yang, Y.; Lee, M.S.; Lee, H.G.; Ryu, J.H.; Lim, J.S. Antimelanogenic effect of 4-hydroxylonchocarpin through the inhibition of tyrosinase-related proteins and MAPK phosphatase. Exp. Dermatol. 2016, 25, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Nakamura, R.; Baba, K. Beneficial effect of laserpitin, a coumarin compound from Angelica keiskei, on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2005, 32, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sawada, K.; Yamamoto, N.; Ashida, H. 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadiopocytes to adipocytes via AMPK and MAPK pathways. Mol. Nutr. Food Res. 2013, 57, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed-Aly, M.R.; Abd-El-Razek-Fodah, H.H.; Saleh, S.Y. Antiobesity, antioxidant and cytotoxicity activities of newly synthesized chalcone derivatives and their metal complexes. Eur. J. Med. Chem. 2014, 76, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Katsori, A.M.; Hadjipavlou-Litina, D. Recent progress in therapeutic applications of chalcones. Expert Opin. Ther. Pat. 2011, 21, 1575–1596. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Sandjo, L.P. Isobavachalcone: An overview. Chin. J. Integr. Med. 2012, 18, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Xu, B.; Zeng, G.; Tan, J.; He, X.; Hu, C.; Zhou, Y. Chemical constituents of Morus alba L. and their inhibitory effect on 3T3-L1 preadipocyte proliferation and differentiation. Fitoterapia 2014, 98, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Fernández-Real, J. M. Adipocyte differentiation. In Adipose Tissue Biology; Springer: Basel, Switzerland, 2017; Volume 6, pp. 69–90. [Google Scholar]
- Choi, K.M.; Lee, Y.S.; Sin, D.M.; Lee, S.; Lee, M.K.; Lee, Y.M.; Hong, J.T.; Yun, Y.P.; Yoo, H.S. Sulforaphane inhibits mitotic clonal expansion during adipogenesis through cell cycle arrest. Obesity 2012, 20, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Shinoda, A.; Furuya, N.; Harada, E.; Arimura, N.; Ichi, I.; Fujiwara, Y.; Inoue, J.; Sato, R. Perilipin-mediated lipid droplet formation in adipocytes promotes sterol regulatory element-binding protein-1 processing and triacylglyceride accumulation. PLoS ONE 2013, 8, e64605. [Google Scholar] [CrossRef] [PubMed]
- Novikoff, A.B.; Novikoff, P.M.; Rosen, O.M.; Rubin, C.S. Organelle relationships in cultured 3T3-L1 preadipocytes. J. Cell Biol. 1980, 87, 180–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Xu, J.; Zhang, X.; Yang, J.; Zhang, D.; Huang, J.; Lv, P.; Shen, W.; Yang, Y. Berberine attenuates autophagy in adipocytes by targeting BECN1. Autophagy 2014, 10, 1776–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulos, S.P.; Dodson, M.V.; Hausman, G.J. Cell line models for differentiation: Preadipocytes and adipocytes. Exp. Biol. Med. 2010, 235, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.J.; Kim, J.H.; Jeong, H.Y.; Ji, H.H.; Nam, S.W.; Lee, E.W.; Kim, B.W.; Kwon, H.J. Widdrol blocks 3T3-L1 preadipocytes growth and differentiation due to inhibition of mitotic clonal expansion. J. Microbiol. Biotechnol. 2012, 22, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Chauhan, P.; Varshney, S.; Shankar, K.; Rajan, S.; Saini, D.; Srivastava, M.; Yadav, P.P.; Gaikwad, A.N. A withanolide coagulin-L inhibits adipogenesis modulating Wnt/β-catenin pathway and cell cycle in mitotic clonal expansion. Phytomedicine 2014, 21, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.; Shankar, K.; Beg, M.; Balaramnavar, V.M.; Mishra, S.K.; Jagdale, P.; Srivastava, S.; Chhonker, Y.S.; Lakshmi, V.; Chaudhari, B.P. Rohitukine inhibits in vitro adipogenesis arresting mitotic clonal expansion and improves dyslipidemia in vivo. J. Lipid Res. 2014, 55, 1019–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.L.; Lin, Y.J.; Ho, C.T.; Yen, G.C. Inhibitory effects of garcinol and pterostilbene on cell proliferation and adipogenesis in 3T3-L1 cells. Food Funct. 2012, 3, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Nam, H.; Morrison, R.F. Curcumin inhibits 3T3-L1 preadipocyte proliferation by mechanisms involving post-transcriptional p27 regulation. Biochem. Biophys. Rep. 2016, 5, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Mitterberger, M.C.; Zwerschke, W. Mechanisms of resveratrol-induced inhibition of clonal expansion and terminal adipogenic differentiation in 3T3-L1 preadipocytes. J. Gerontol. 2013, 68, 1356–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Klemm, D.J.; Vinson, C.; Lane, M.D. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J. Biol. Chem. 2004, 279, 4471–4478. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guichard, C.; Ferré, P.; Foufelle, F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc. Natl. Acad. Sci. USA 1999, 96, 12737–12742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Kim, J.; Park, J.Y.; Kang, K.S.; Park, J.H.; Hwang, G.S. Processed Panax ginseng, sun ginseng, inhibits the differentiation and proliferation of 3T3-L1 preadipocytes and fat accumulation in Caenorhabditis elegans. J. Ginseng Res. 2017, 41, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordonier, E.L.; Jarecke, S.K.; Hollinger, F.E.; Zempleni, J. Inhibition of acetyl-CoA carboxylases by soraphen A prevents lipid accumulation and adipocyte differentiation in 3T3-L1 cells. Eur. J. Pharmacol. 2016, 780, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Sung, S.H. Platyphylloside isolated from Betula platyphylla inhibit adipocyte differentiation and induce lipolysis via regulating adipokines including PPARγ in 3t3-l1 cells. Pharmacog. Mag. 2016, 12, 276–281. [Google Scholar]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.; Van Essen, P.; Koenen, T.; Joosten, L.; Netea, M.; Tack, C.; Stienstra, R. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology 2012, 153, 5866–5874. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, C.; Rodrigues, V.; Gomes, F.; De Moura, R.; Victorio, S.; Bombassaro, B.; Chaim, E.; Pareja, J.; Geloneze, B.; Velloso, L. Defective regulation of adipose tissue autophagy in obesity. Int. J. Obes. 2013, 37, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Baerga, R.; Zhang, Y.; Chen, P.-H.; Goldman, S.; Jin, S.V. Targeted deletion of autophagy-related 5 (Atg5) impairs adipogenesis in a cellular model and in mice. Autophagy 2009, 5, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Goldman, S.; Baerga, R.; Zhao, Y.; Komatsu, M.; Jin, S. Adipose-specific deletion of autophagy-related gene 7 (Atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 19860–19865. [Google Scholar] [CrossRef] [PubMed]
- Skop, V.; Cahova, M.; Dankova, H.; Papackova, Z.; Palenickova, E.; Svoboda, P.; Zidkova, J.; Kazdova, L. Autophagy inhibition in early but not in later stages prevents 3T3-L1 differentiation: Effect on mitochondrial remodeling. Differentiation 2014, 87, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I. Autophagosome formation and molecular mechanism of autophagy. Antioxid. Redox Signal. 2011, 14, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, Y.; Okutsu, M.; Ong, L.C.; Jin, Y.; Zheng, L.; Chow, P.; Yu, S.; Zhang, M.; Yan, Z. Autophagy is involved in adipogenic differentiation by repressesing proteasome-dependent PPARγ2 degradation. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E530–E539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakifahmetoglu-Norberg, H.; Xia, H.G.; Yuan, J. Pharmacologic agents targeting autophagy. J. Clin. Investig. 2015, 125, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Broeder, M.J.; Kopylova, V.A.; Kamminga, L.M.; Legler, J. Zebrafish as a model to study the role of peroxisome proliferating-activated receptors in adipogenesis and obesity. PPAR Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Farber, S.A.; Pack, M.; Ho, S.Y.; Johnson, I.D.; Wagner, D.S.; Dosch, R.; Mullins, M.C.; Hendrickson, H.S.; Hendrickson, E.K.; Halpern, M.E. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science 2001, 292, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Thorpe, J.L.; Deng, Y.; Santana, E.; DeRose, R.A.; Farber, S.A. Lipid metabolism in zebrafish. Methods Cell Biol. 2004, 76, 87–108. [Google Scholar] [PubMed]
- Kim, J.H.; Kang, S.; Jung, Y.N.; Choi, H.S. Cholecalciferol inhibits lipid accumulation by regulating early adipogenesis in cultured adipocytes and zebrafish. Biochem. Biophys. Res. Commun. 2016, 469, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Obiang-Obounou, B.W.; Lee, J.; Lee, T.Y.; Bae, M.A.; Hwang, K.S.; Lee, K.B.; Choi, J.S.; Jang, B.C. Anti-adipogenic effects on 3t3-l1 cells and zebrafish by tanshinone IIA. Int. J. Mol. Sci. 2017, 18, 2065. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Sahin, K.; Bilen, H.; Bahcecioglu, I.H.; Bilir, B.; Ashraf, S.; Halazun, K.J.; Kucuk, O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 161–171. [Google Scholar] [PubMed]
- Kim, D.H.; Li, H.; Han, Y.E.; Jeong, J.H.; Lee, H.J.; Ryu, J.H. Modulation of inducible nitric oxide synthase expression in lps-stimulated bv-2 microglia by prenylated chalcones from Cullen corylifolium (L.) medik. through inhibition of I-kappabalpha degradation. Molecules 2018, 23, 109. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish Danio Rerio, 4th ed.; University of Otegon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Clifton, J.D.; Lucumi, E.; Myers, M.C.; Napper, A.; Hama, K.; Farber, S.A.; Smith, A.B.; Huryn, D.M.; Diamond, S.L.; Pack, M. Identification of novel inhibitors of dietary lipid absorption using zebrafish. PLoS ONE 2010, 5, e12386. [Google Scholar] [CrossRef] [PubMed]
- Progatzky, F.; Sangha, N.J.; Yoshida, N.; McBrien, M.; Cheung, J.; Shia, A.; Scott, J.; Marchesi, J.R.; Lamb, J.R.; Bugeon, L.; et al. Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat. Commun. 2014, 5, 5864. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Gene Name | Forward Primer | Reverse Primer | Accession Number |
|---|---|---|---|
| PPARγ | AACTCTGGGAGATTCTCCTGTTGA | GAAGTGCTCATAGGCAGTGCAT | EF062476 |
| PPARγ(Zf) | AGTACGGGGTCATCGAAGTG | GCGCAGACTCTTGAGGAACT | NM131467.1 |
| C/EBPα | TGCACCACCAACTGCTTAG | AAACCATCCTCTGGGTCTCC | NM001287523 |
| C/EBPα(Zf) | CATCGACATCAGCGCCTACA | CACCGTGGTGGTAGTCGTAG | NC007118.7 |
| SREBP1c | TGTTGGCATCCTGCTATCTG | AGGGAAAGCTTTGGGGTCTA | XP011247147. |
| Adiponectin | TGTAGGATTGTCAGTGGATCTG | GCTCTTCAGTTGTAGTAACGTCATC | NP033735.3 |
| FAS | AGCGGCCATTTCCATTGCCC | CCATGCCCAGAGGGTGGTTG | NP032014.3 |
| ACC1 | GTCAGCGGATGGGCGGAATG | CGCCGGATGCCATGCTCAAC | XP011247147.1 |
| BECN1 | ACCGGGTCACCATCCAGGAA | GAAGCTATTAGCACTTTCTGT | NP062530.2 |
| Atg5 | TGTGCTTCGAGATGTGTGGTT | GTCAAATAGCTGACTCTTGGCAA | NP444299.1 |
| Atg7 | CCTGCACAACACCAACACAC | CACCTGACTTTATGGCTTCCC | NP001240646 |
| GAPDH | TGCACCACCAACTGCTTAG | GGCATGGACTGTGGTCATGAG | BC096042 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Li, H.; Kweon, M.; Choi, Y.; Kim, M.J.; Ryu, J.-H. Isobavachalcone from Angelica keiskei Inhibits Adipogenesis and Prevents Lipid Accumulation. Int. J. Mol. Sci. 2018, 19, 1693. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061693
Lee H, Li H, Kweon M, Choi Y, Kim MJ, Ryu J-H. Isobavachalcone from Angelica keiskei Inhibits Adipogenesis and Prevents Lipid Accumulation. International Journal of Molecular Sciences. 2018; 19(6):1693. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061693
Chicago/Turabian StyleLee, Hyejin, Hua Li, Minson Kweon, Youngsook Choi, Min Jung Kim, and Jae-Ha Ryu. 2018. "Isobavachalcone from Angelica keiskei Inhibits Adipogenesis and Prevents Lipid Accumulation" International Journal of Molecular Sciences 19, no. 6: 1693. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061693