Chrysin Attenuates Cell Viability of Human Colorectal Cancer Cells through Autophagy Induction Unlike 5-Fluorouracil/Oxaliplatin
Abstract
:1. Introduction
2. Results
2.1. Chrysin Achieved Similar Effectiveness to 5-Fluorouracil (5-FU) Combined with Oxaliplatin in a Panel of Colorectal Cancer (CRC) Cells
2.2. LC3-II Levels Were Induced in Chrysin-Treated but Not 5-Fluorouracil (5-FU) /Oxaliplatin-Treated Colorectal Cancer (CRC) Cells
2.3. Blockage of Autophagy Induction Restored Chrysin-Attenuated Colorectal Cancer (CRC) Cell Viability
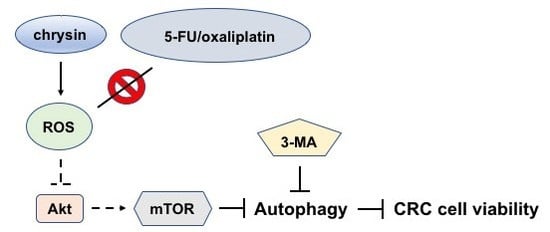
2.4. Chrysin-Mediated Autophagy Induction Was through Protein Kinase B (Akt)/Mammalian Target of Rapamycin (mTOR) Signaling Pathway in Colorectal Cancer (CRC) Cells
2.5. Chrysin Induced the Production of Reactive Oxygen Species (ROS) in Colorectal Cancer (CRC) Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Cell Viability Assay
4.3. Immunofluorescence Staining
4.4. Detection of Intracellular Reactive Oxygen Species (ROS)
4.5. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| CRC | colorectal cancer |
| COX-2 | cyclooxygenase-2 |
| PPARγ | peroxisome proliferator-activated receptor-γ |
| cleaved-PARP | cleaved poly-ADP-ribose polymerase |
| 3-MA | 3-methyladenine |
| ROS | reactive oxygen species |
| BCRP | breast cancer resistance protein |
| ABC | ATP-binding cassette |
| MDR | multidrug resistance |
| PBS | phosphate buffered saline |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| AMPKα | AMP-activated protein kinase alpha |
| mTOR | mammalian target of rapamycin |
References
- Puccini, A.; Berger, M.D.; Naseem, M.; Tokunaga, R.; Battaglin, F.; Cao, S.; Hanna, D.L.; McSkane, M.; Soni, S.; Zhang, W.; et al. Colorectal cancer: Epigenetic alterations and their clinical implications. Biochim. Biophys. Acta 2017, 1868, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Mansoori, B.; Baradaran, B. The role of micrornas in colorectal cancer. Biomed. Pharmacother. 2016, 84, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Coppede, F.; Lopomo, A.; Spisni, R.; Migliore, L. Genetic and epigenetic biomarkers for diagnosis, prognosis and treatment of colorectal cancer. World J. Gastroenterol. 2014, 20, 943–956. [Google Scholar] [CrossRef] [PubMed]
- De Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Tournigand, C.; Andre, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. Folfiri followed by folfox6 or the reverse sequence in advanced colorectal cancer: A randomized gercor study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Field, K.M.; Kosmider, S.; Jefford, M.; Jennens, R.; Green, M.; Gibbs, P. Chemotherapy treatments for metastatic colorectal cancer: Is evidence-based medicine in practice? J. Oncol. Pract. 2008, 4, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. 2014, 20, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Prochilo, T.; Abeni, C.; Bertocchi, P.; Zaniboni, A. Oxaliplatin-induced lung toxicity. Case report and review of the literature. Curr. Drug Saf. 2012, 7, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Huang, M.; Wang, Y.; Wang, L.; Chen, C.; Chu, X. Micrornas as regulators, biomarkers and therapeutic targets in the drug resistance of colorectal cancer. Cell. Physiol. Biochem. 2016, 40, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Temraz, S.; Mukherji, D.; Alameddine, R.; Shamseddine, A. Methods of overcoming treatment resistance in colorectal cancer. Crit. Rev. Oncol. Hematol. 2014, 89, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Nobili, S.; Paolicchi, E.; Perrone, G.; Napoli, C.; Landini, I.; Danesi, R.; Mini, E. Epigenetics and chemoresistance in colorectal cancer: An opportunity for treatment tailoring and novel therapeutic strategies. Drug Resist. Updates 2011, 14, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Sakurai, H.; Abdelhamed, S.; Yokoyama, S.; Maruyama, T.; Athikomkulchai, S.; Viriyaroj, A.; Awale, S.; Yagita, H.; Ruchirawat, S.; et al. A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells. Oncol. Rep. 2013, 30, 2357–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid.-Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Qin, H.; Shi, Q.; Zhang, Y.; Zhou, F.; Wu, H.; Ding, S.; Niu, Z.; Lu, Y.; Shen, P. Chrysin attenuates inflammation by regulating m1/m2 status via activating PPARγ. Biochem. Pharmacol. 2014, 89, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.J.; Jeong, Y.J.; Inoue, H.; Park, J.W.; Kwon, T.K. Chrysin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression through the inhibition of nuclear factor for IL-6 (NF-IL6) DNA-binding activity. FEBS Lett. 2005, 579, 705–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koosha, S.; Alshawsh, M.A.; Looi, C.Y.; Seyedan, A.; Mohamed, Z. An association map on the effect of flavonoids on the signaling pathways in colorectal cancer. Int. J. Med. Sci. 2016, 13, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Loffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-ULK1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol. 2009, 196, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. Ros signaling under metabolic stress: Cross-talk between ampk and akt pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Heras-Sandoval, D.; Perez-Rojas, J.M.; Hernandez-Damian, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jing, K.; Jeong, S.; Kim, N.; Song, K.S.; Heo, J.Y.; Park, J.H.; Seo, K.S.; Han, J.; Park, J.I.; et al. The omega-3 polyunsaturated fatty acid dha induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated AKT-mTOR signaling in prostate cancer cells expressing mutant p53. BioMed Res. Int. 2013, 2013, 568671. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Kou, J.; Han, X.; Li, X.; Zhong, Z.; Liu, Z.; Zheng, Y.; Tian, Y.; Yang, L. ROS-dependent activation of autophagy through the PI3K/AKT/mTOR pathway is induced by hydroxysafflor yellow a-sonodynamic therapy in THP-1 macrophages. Oxid. Med. Cell. Longev. 2017, 2017, 8519169. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Park, K.I.; Kim, S.H.; Yu, S.N.; Park, S.G.; Kim, Y.W.; Seo, Y.K.; Ma, J.Y.; Ahn, S.C. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int. J. Mol. Sci. 2017, 18, 1088. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell. Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Roudkenar, M.H.; Seydi, E.; Sadeghi, L.; Mohseni, A.; Pirahmadi, N.; Pourahmad, J. Chrysin as an anti-cancer agent exerts selective toxicity by directly inhibiting mitochondrial complex II and V in CLL B-lymphocytes. Cancer Investig. 2017, 35, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Goldman, B. Multidrug resistance: Can new drugs help chemotherapy score against cancer? J. Natl. Cancer Inst. 2003, 95, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Das, A.S.; Majumder, M.; Mukhopadhyay, R. Antiatherogenic roles of dietary flavonoids chrysin, quercetin, and luteolin. J. Cardiovasc. Pharmacol. 2016, 68, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Rezaee, S.A.; Hosseinzadeh, H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed. Pharmacother. 2017, 92, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. Abc multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jin, J.; Yu, H.; Zhao, Z.; Ma, D.; Zhang, C.; Jiang, H. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J. Exp. Clin. Cancer Res. 2017, 36, 44. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Chen, A.L.; Hung, H.C.; Chien, Y.H.; Huang, J.S.; Huang, C.Y.; Chen, Y.W.; Chen, C.N. Chrysin: A histone deacetylase 8 inhibitor with anticancer activity and a suitable candidate for the standardization of chinese propolis. J. Agric. Food Chem. 2012, 60, 11748–11758. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Xue, J.; Li, Z.; Shi, X.; Jiang, B.H.; Fang, J. Chrysin inhibits expression of hypoxia-inducible factor-1α through reducing hypoxia-inducible factor-1α stability and inhibiting its protein synthesis. Mol. Cancer Ther. 2007, 6, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Otake, Y.; Brubaker, J.A.; Walle, U.K.; Halushka, P.V. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br. J. Clin. Pharmacol. 2001, 51, 143–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, P.G. Developmental cell death: Morphological diversity and multiple mechanisms. Anat. Embryol. 1990, 181, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.L.; Chen, C.M.; Chang, Y.Z.; Liu, G.Y.; Hung, H.C.; Hsieh, T.Y.; Lin, C.L. Pine (pinus morrisonicola hayata) needle extracts sensitize GBM8901 human glioblastoma cells to temozolomide by downregulating autophagy and O6-methylguanine-DNA methyltransferase expression. J. Agric. Food Chem. 2014, 62, 10458–10467. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, F.M.; Kucukler, S.; Eldutar, E.; Caglayan, C.; Gulcin, I. Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy: A multi-biomarker approach. Sci. Pharm. 2017, 85, 4. [Google Scholar] [CrossRef] [PubMed]
- Swart, C.; Du Toit, A.; Loos, B. Autophagy and the invisible line between life and death. Eur. J. Cell Biol. 2016, 95, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Fu, Y.S.; Tsai, M.J.; Cheng, H.; Weng, C.F. Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. Int. J. Mol. Sci. 2017, 18, E1412. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-M.; Chen, C.-I.; Hsiang, Y.-P.; Hsu, Y.-C.; Cheng, K.-C.; Chien, P.-H.; Pan, H.-L.; Lu, C.-C.; Chen, Y.-J. Chrysin Attenuates Cell Viability of Human Colorectal Cancer Cells through Autophagy Induction Unlike 5-Fluorouracil/Oxaliplatin. Int. J. Mol. Sci. 2018, 19, 1763. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061763
Lin Y-M, Chen C-I, Hsiang Y-P, Hsu Y-C, Cheng K-C, Chien P-H, Pan H-L, Lu C-C, Chen Y-J. Chrysin Attenuates Cell Viability of Human Colorectal Cancer Cells through Autophagy Induction Unlike 5-Fluorouracil/Oxaliplatin. International Journal of Molecular Sciences. 2018; 19(6):1763. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061763
Chicago/Turabian StyleLin, Yueh-Ming, Chih-I Chen, Yi-Ping Hsiang, Yung-Chia Hsu, Kung-Chuan Cheng, Pei-Hsuan Chien, Hsiao-Lin Pan, Chien-Chang Lu, and Yun-Ju Chen. 2018. "Chrysin Attenuates Cell Viability of Human Colorectal Cancer Cells through Autophagy Induction Unlike 5-Fluorouracil/Oxaliplatin" International Journal of Molecular Sciences 19, no. 6: 1763. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061763