The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Treatment with Olive Biophenols Reduces Pancreatic Cancer Cell Viability
2.2. Oleuropein and Hydroxytyrosol Induce Morphological Changes in Pancreatic Cells
2.3. Olive Biophenols Cause G2/M Cell Cycle Arrest in Pancreatic Cells
2.4. Treatment with Oleuropein and Hydroxytyrosol Promotes Caspase 3/7 Dependent Apoptosis
2.5. Differential Expression of Bcl2 Family Proteins Following Treatment with Oleuropein and Hydroxytyrosol
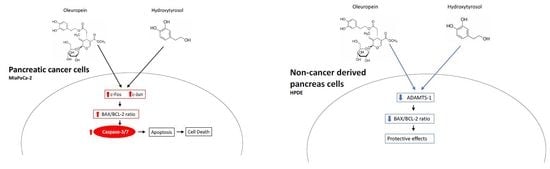
2.6. Gene and Protein Expression Changes in MIA PaCa-2 Cells Following Treatment with Oleuropein and Hydroxytyrosol
2.7. Reduced Expression of ADAMTS1 in HPDE Cells Following Treatment with Oleuropein
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Pancreas Cell Culture
4.3. Assessment of Cell Growth Inhibition
4.4. Apoptosis Assay
4.5. Cell Cycle Analysis
4.6. Assessment of the Effect of Oleuropein and Hydroxytyrosol on Gene Expression
4.7. Protein Expression
4.8. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 869–873. [Google Scholar] [PubMed]
- Fortes, C.; Forastiere, F.; Farchi, S.; Mallone, S.; Trequattrinni, T.; Anatra, F.; Schmid, G.; Perucci, C.A. The protective effect of the Mediterranean diet on lung cancer. Nutr. Cancer 2003, 46, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kapiszewska, M.; Soltys, E.; Visioli, F.; Cierniak, A.; Zajac, G. The protective ability of the Mediterranean plant extracts against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. J. Physiol. Pharmacol. 2005, 56 (Suppl. 1), 183–197. [Google Scholar] [PubMed]
- Bosetti, C.; Turati, F.; Dal Pont, A.; Ferraroni, M.; Polesel, J.; Negri, E.; Serraino, D.; Talamini, R.; La Vecchia, C.; Zeegers, M.P. The role of Mediterranean diet on the risk of pancreatic cancer. Br. J. Cancer 2013, 109, 1360–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra-Majem, L.; Ngo de la Cruz, J.; Ribas, L.; Tur, J.A. Olive oil and the Mediterranean diet: Beyond the rhetoric. Eur. J. Clin. Nutr. 2003, 57 (Suppl. 1), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Burns, C. The Australian Olive Industry Research, Development and Extension Plan 2010–2015; RIRDC, Ed.; RIRDC: Canberra, Australia, 2010; pp. 1–76. [Google Scholar]
- Goldsmith, C.D.; Stathopoulos, C.E.; Golding, J.B.; Roach, P.D. Fate of phenolic compounds during olive oil production with the traditional press method. Int. Food Res. J. 2014, 21, 101–109. [Google Scholar]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Investigation of Australian olive mill waste for recovery of biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920. [Google Scholar] [CrossRef] [PubMed]
- Seabra, R.M.; Vinha, A.F.; Ferreres, F.; Silva, B.M.; Valentao, P.; Goncalves, A.; Pereira, J.A.; Oliveira, M.B.; Andrade, P.B. Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influences of cultivar and geographical origin. Food Chem. 2005, 89, 561–568. [Google Scholar] [CrossRef]
- Goldsmith, C.; Vuong, Q.; Sadeqzadeh, E.; Stathopoulos, C.; Roach, P.; Scarlett, C. Phytochemical Properties and Anti-Proliferative Activity of Olea europaea L. Leaf Extracts against Pancreatic Cancer Cells. Molecules 2015, 20, 12992–13004. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Exarchou, V.; Troganis, A.N.; Psomiadou, E.; Fotsis, T.; Briasoulis, E.; Gerothanassis, I.P. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol. Nutr. Food Res. 2009, 53, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.-H.; Lee, B.-Y.; Lee, J.; Lee, H.-B.; Son, J.-Y.; Park, C.-S.; Shetty, K.; Kim, Y.-C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Gomez Caravaca, A.M.; Carrasco Pancorbo, A.; Canabate Diaz, B.; Segura Carretero, A.; Fernandez Gutierrez, A. Electrophoretic identification and quantitation of compounds in the polyphenolic fraction of extra-virgin olive oil. Electrophoresis 2005, 26, 3538–3551. [Google Scholar] [CrossRef] [PubMed]
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; Mayas, M.D.; Martínez-Martos, J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci. Technol. 2013, 31, 92–99. [Google Scholar] [CrossRef]
- Klen, T.J.; Vodopivec, B.M. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two- and three-phase centrifuge. LWT Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Elamin, M.H.; Omer, S.A.; Daghestani, M.H.; Al-Olayan, E.S.; Elobeid, M.A.; Virk, P. Oleuropein induces apoptosis via the p53 pathway in breast cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 6739–6742. [Google Scholar] [CrossRef]
- Cardeno, A.; Sanchez-Hidalgo, M.; Rosillo, M.A.; de la Lastra, C.A. Oleuropein, a secoiridoid derived from olive tree, inhibits the proliferation of human colorectal cancer cell through downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Galvano, F.; Santangelo, R.; Cardile, V.; Gangia, S.; D’Orazio, N.; Abraham, N.G.; Vanella, L. Antiproliferative effect of oleuropein in prostate cell lines. Int. J. Oncol. 2012, 41, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.M.; Chai, E.Q.; Cai, H.Y.; Miao, G.Y.; Ma, W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Montedoro, G.F.; Morozzi, G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur. J. Cancer Prev. 2002, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Araki, M.; Kusunoki, M.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: Role of Nrf2 activation and HO-1 induction. J. Agric. Food Chem. 2011, 59, 4473–4482. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.H.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; Elkhadragy, M.F. Synergistic Anti-Breast-Cancer Effects of Combined Treatment With Oleuropein and Doxorubicin In Vivo. Altern. Ther. Health Med. 2017, 23, 7. [Google Scholar]
- Samara, P.; Christoforidou, N.; Lemus, C.; Argyropoulou, A.; Ioannou, K.; Vougogiannopoulou, K.; Aligiannis, N.; Paronis, E.; Gaboriaud-Kolar, N.; Tsitsilonis, O.; et al. New semi-synthetic analogs of oleuropein show improved anticancer activity in vitro and in vivo. Eur. J. Med. Chem. 2017, 137, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M. Olive leaf extract and its main component oleuropein prevent chronic ultraviolet B radiation-induced skin damage and carcinogenesis in hairless mice. J. Nutr. 2009, 139, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, Z.; Ma, Y.; Song, R.; Pei, T.; Zheng, T.; Wang, J.; Xu, D.; Fang, X.; Jiang, H.; et al. Hydroxytyrosol inhibits cholangiocarcinoma tumor growth: An in vivo and in vitro study. Oncol. Rep. 2014, 31, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Bosetti, C.; Bravi, F.; Turati, F.; Edefonti, V.; Polesel, J.; Decarli, A.; Negri, E.; Talamini, R.; Franceschi, S.; La Vecchia, C.; Zeegers, M.P. Nutrient-based dietary patterns and pancreatic cancer risk. Ann. Epidemiol. 2013, 23, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Mandavilli, B.S.; Yan, M.; Clarke, S. Cell-Based High Content Analysis of Cell Proliferation and Apoptosis. Methods Mol. Biol. 2018, 1683, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Thandapani, P.; Aifantis, I. Apoptosis, Up the Ante. Cancer Cell 2017, 32, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Husain, K.; Zhang, A.; Centeno, B.A.; Chen, D.-T.; Tong, Z.; Sebti, S.M.; Malafa, M.P. EGR-1/Bax pathway plays a role in vitamin E δ-tocotrienol-induced apoptosis in pancreatic cancer cells. J. Nutr. Biochem. 2015, 26, 797–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Zhao, W.; Du, Y.; Zhang, T.; You, L.; Zhao, Y. Activator protein 1 promotes gemcitabine-induced apoptosis in pancreatic cancer by upregulating its downstream target Bim. Oncol. Lett. 2016, 12, 4732–4738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Arao Tan, I.; Ricciardelli, C.; Russell, D.L. The metalloproteinase ADAMTS1: A comprehensive review of its role in tumorigenic and metastatic pathways. Int. J. Cancer 2013, 133, 2263–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Oleuropein in Olive and its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimento, A.; Casaburi, I.; Rosano, C.; Avena, P.; De Luca, A.; Campana, C.; Martire, E.; Santolla, M.F.; Maggiolini, M.; Pezzi, V.; et al. Oleuropein and hydroxytyrosol activate GPER/GPR30-dependent pathways leading to apoptosis of ER-negative SKBR3 breast cancer cells. Mol. Nutr. Food Res. 2013, 58, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Gennari, L.; Blasa, M.; Selvaggini, R.; Urbani, S.; Esposto, S.; Servili, M.; Ninfali, P. Chemical and cellular antioxidant activity of phytochemicals purified from olive mill waste waters. J. Agric. Food Chem. 2011, 59, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Secme, M.; Eroglu, C.; Dodurga, Y.; Bagci, G. Investigation of anticancer mechanism of oleuropein via cell cycle and apoptotic pathways in SH-SY5Y neuroblastoma cells. Gene 2016, 585, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S.K.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Hydroxytyrosol Induces Apoptosis and Cell Cycle Arrest and Suppresses Multiple Oncogenic Signaling Pathways in Prostate Cancer Cells. Nutr. Cancer 2017, 69, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Baudino, T.A. Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr. Drug Discov. Technol. 2015, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Colombini, M. Regulation of ceramide channels by Bcl-2 family proteins. FEBS Lett. 2010, 584, 2128–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Zhu, X.; Du, L. P38 MAP kinase is involved in oleuropein-induced apoptosis in A549 cells by a mitochondrial apoptotic cascade. Biomed. Pharmacother. 2017, 95, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.K.; Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Al-Olayan, E.M.; Elobeid, M.A.; Virk, P.; Mohammed, O.B. Oleuropein induces anti-metastatic effects in breast cancer. Asian Pac. J. Cancer Prev. APJCP 2012, 13, 4555–4559. [Google Scholar] [CrossRef] [PubMed]
- Chinenov, Y.; Kerppola, T.K. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 2001, 20, 2438–2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaulian, E. AP-1—The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell. Signal. 2010, 22, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Babij, C.; Whitfield, J.; Pfarr, C.M.; Lallemand, D.; Yaniv, M.; Rubin, L.L. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron 1995, 14, 927–939. [Google Scholar] [CrossRef]
- Estus, S.; Zaks, W.J.; Freeman, R.S.; Gruda, M.; Bravo, R.; Johnson, E.M., Jr. Altered gene expression in neurons during programmed cell death: Identification of c-jun as necessary for neuronal apoptosis. J. Cell Biol. 1994, 127, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Oben, K.Z.; Alhakeem, S.S.; McKenna, M.K.; Brandon, J.A.; Mani, R.; Noothi, S.K.; Jinpeng, L.; Akunuru, S.; Dhar, S.K.; Singh, I.P.; et al. Oxidative stress-induced JNK/AP-1 signaling is a major pathway involved in selective apoptosis of myelodysplastic syndrome cells by Withaferin-A. Oncotarget 2017, 8, 77436–77452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaiselvan, I.; Samuthirapandi, M.; Govindaraju, A.; Sheeja Malar, D.; Kasi, P.D. Olive oil and its phenolic compounds (hydroxytyrosol and tyrosol) ameliorated TCDD-induced heptotoxicity in rats via inhibition of oxidative stress and apoptosis. Pharm. Biol. 2016, 54, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Hassanshahi, J.; Soleimani, M.; Zamani, F. Neuroprotective effect of olive oil in the hippocampus CA1 neurons following ischemia: Reperfusion in mice. J. Neurosci. Rural Pract. 2013, 4, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Geyikoglu, F.; Emir, M.; Colak, S.; Koc, K.; Turkez, H.; Bakir, M.; Hosseinigouzdagani, M.; Cerig, S.; Keles, O.N.; Ozek, N.S. Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury. J. Food Drug Anal. 2017, 25, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Kuno, K.; Okada, Y.; Kawashima, H.; Nakamura, H.; Miyasaka, M.; Ohno, H.; Matsushima, K. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett. 2000, 478, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Gustavsson, H.; Wang, W.; Jennbacken, K.; Welen, K.; Damber, J.E. ADAMTS1, a putative anti-angiogenic factor, is decreased in human prostate cancer. BJU Int. 2009, 104, 1786–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, G.E.; Kleivi, K.; Meling, G.I.; Teixeira, M.R.; Thiis-Evensen, E.; Rognum, T.O.; Lothe, R.A. ADAMTS1, CRABP1, and NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell. Oncol. 2006, 28, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kim, D.S.; Kim, E.J.; Chae, M.H.; Cha, S.I.; Kim, C.H.; Jheon, S.; Jung, T.H.; Park, J.Y. Aberrant methylation of ADAMTS1 in non-small cell lung cancer. Cancer Genet. Cytogenet. 2008, 187, 80–84. [Google Scholar] [CrossRef] [PubMed]






| Cell Line | Oleuropein (µM) | Hydroxytyrosol (µM) | Tyrosol (µM) | Gemcitabine (nM) |
|---|---|---|---|---|
| MIA PaCa-2 | 150.1 | 75.1 | >300 | 31.02 |
| BxPC-3 | >300 | >300 | >300 | 3.6 |
| CFPAC-1 | >300 | >300 | >300 | 2.6 |
| ASPC-1 | >300 | >300 | >300 | 12 |
| HPDE | >300 | 65.5 | >300 | 0.04 |
| Cell Line | Gene Symbol | Treatment | Fold Change (Linear) (VS. Control) | ANOVA p-Value (VS. Control) |
|---|---|---|---|---|
| MIA PaCa-2 | JUN | Oleuropein | 4.64 | 0.000126 |
| Hydroxytyrosol | 4.68 | 0.000041 | ||
| FOS | Oleuropein | 2.41 | 0.007736 | |
| Hydroxytyrosol | 4.98 | 0.000103 | ||
| EGR-1 | Oleuropein | 8.01 | 0.00083 | |
| Hydroxytyrosol | 20.75 | 0.000019 | ||
| HPDE | ADAMTS1 | Oleuropein | −2.19 | 0.00003 |
| Hydroxytyrosol | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19071937
Goldsmith CD, Bond DR, Jankowski H, Weidenhofer J, Stathopoulos CE, Roach PD, Scarlett CJ. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. International Journal of Molecular Sciences. 2018; 19(7):1937. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19071937
Chicago/Turabian StyleGoldsmith, Chloe D., Danielle R. Bond, Helen Jankowski, Judith Weidenhofer, Costas E. Stathopoulos, Paul D. Roach, and Christopher J. Scarlett. 2018. "The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells" International Journal of Molecular Sciences 19, no. 7: 1937. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19071937