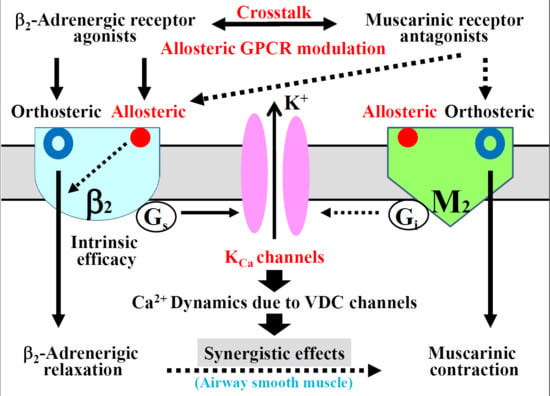
Involvement of Allosteric Effect and KCa Channels in Crosstalk between β2-Adrenergic and Muscarinic M2 Receptors in Airway Smooth Muscle
Abstract
:1. Introduction
2. Results
2.1. Synergism in the Combination of β2-Adrenergic Receptors with Muscarinic Receptor Antagonists
2.2. Role of G Protein/Ca2+-Activated K+ Channel Linkage in the Synergistic Effects
2.3. Role of Orthosteric Sites in the Effects of Muscarinic Receptor Antagonists
2.4. Role of Allosteric Sites in the Effects of β2-Adrenergic Receptor Agonists
2.5. Role of Allosteric Effects in the Synergistic Effects of β2-Adrenergic Receptor Agonists with Muscarinic Receptor Antagonists
2.6. Possible Mechanisms of cAMP-Independent Processes in the Synergistic Effect
2.7. Possible Involvement of Interaction between Muscarinic Receptor Antagonists and Allosteric Sites on β2-Adrenergic Receptors
3. Discussion
4. Materials and Methods
4.1. Tissue Preparation and Tension Records
4.2. Analysis of Synergistic Effect
4.3. Experimental Protocols
4.4. Materials
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kume, H.; Graziano, M.P.; Kotlikoff, M.I. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proc. Natl. Acad. Sci. USA 1992, 89, 11051–11055. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Mikawa, K.; Takagi, K.; Kotlikoff, M.I. Role of G proteins and KCa channels in the muscarinic and β-adrenergic regulation of airway smooth muscle. Am. J. Physiol. 1995, 268, L221–L229. [Google Scholar] [CrossRef] [PubMed]
- Kume, H. RhoA/Rho-kinase as a therapeutic target in asthma. Curr. Med. Chem. 2008, 15, 2876–2885. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Fukunaga, K.; Oguma, T. Research and development of bronchodilators for asthma and COPD with a focus on G protein/KCa channel linkage and β2-adrenergic intrinsic efficacy. Pharmacol. Ther. 2015, 156, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kume, H.; Honjo, H.; Katoh, H.; Kodama, I.; Yamaki, K.; Hayashi, H. Possible involvement of Rho kinase in Ca2+ sensitization and mobilization by MCh in tracheal smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L1218–L1224. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Kume, H.; Ito, S.; Oguma, T.; Shiraki, A.; Kondo, M.; Ito, Y.; Shimokata, K. Direct effects of hydrogen peroxide on airway smooth muscle tone: Roles of Ca2+ influx and Rho-kinase. Eur. J. Pharmacol. 2007, 556, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, A.; Kume, H.; Oguma, T.; Makino, Y.; Ito, S.; Shimokata, K.; Honjo, H.; Kamiya, K. Role of Ca2+ mobilization and Ca2+ sensitization in 8-iso-PGF2α-induced contraction in airway smooth muscle. Clin. Exp. Allergy 2009, 39, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sanderson, M.J. The contribution of Ca2+ signaling and Ca2+ sensitivity to the regulation of airway smooth muscle contraction is different in rats and mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L947–L958. [Google Scholar] [CrossRef] [PubMed]
- Mahn, K.; Ojo, O.O.; Chadwick, G.; Aaronson, P.I.; Ward, J.P.; Lee, T.H. Ca2+ homeostasis and structural and functional remodeling of airway smooth muscle in asthma. Thorax 2010, 65, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Kume, H. Ca2+ dynamics and Ca2+ sensitization in the regulation of airway smooth muscle tone. In Muscle Cell and Tissue; Sakuma, K., Ed.; INTECH: Rijeka, Croatia, 2015; pp. 289–330. [Google Scholar]
- Kotlikoff, M.I. Potassium channels in airway smooth muscle: A tale of two channels. Pharmacol. Ther. 1993, 58, 1–12. [Google Scholar] [CrossRef]
- Ghatta, S.; Nimmagadda, D.; Xu, X.; O’Rourke, S.T. Large-conductance calcium-activated potassium channels: Structural and functional implications. Pharmacol. Ther. 2006, 110, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Kume, H. Large-conductance calcium-activated potassium channels. In Calcium Signaling in Airway Smooth Muscle Cells; Wang, Y.X., Ed.; Springer: New York, NY, USA, 2013; pp. 49–83. [Google Scholar]
- Kume, H.; Ito, S. Role of large-conductance calcium-activated potassium channels on airway smooth muscle in physiological and pathological conditions. In Potassium Channels in Health and Disease; Kume, H., Ed.; Nova Science Publishers: New York, NY, USA, 2017; pp. 41–120. [Google Scholar]
- Wang, Y.X.; Fleischmann, B.K.; Kotlikoff, M.I. Modulation of maxi-K+ channels by voltage-dependent Ca2+ channels and methacholine in single airway myocytes. Am. J. Physiol. 1997, 272, C1151–C1159. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Ishikawa, T.; Oguma, T.; Ito, S.; Shimokata, K.; Kotlikoff, M.I. Involvement of Ca2+ mobilization in tachyphylaxis to β-adrenergic receptors in trachealis. Am. J. Respir. Cell Mol. Biol. 2003, 29, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L. Potassium channel diversity in the pulmonary arteries and pulmonary veins: Implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol. Ther. 2007, 115, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.A.; Wang, W.C.; McIlmoyle, E.L.; Robinett, K.S.; Schillinger, R.M.; An, S.S.; Sham, J.S.; Liggett, S.B. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010, 16, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohya, S.; Kito, H.; Hatano, N.; Muraki, K. Recent advances in therapeutic strategies that focus on the regulation of ion channel expression. Pharmacol. Ther. 2016, 160, 11–43. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Matera, M.G.; Cazzola, M. Pharmacological interaction between LABAs and LAMAs in the airways: Optimizing synergy. Eur. J. Pharmacol. 2015, 761, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Trice, J.; Shinde, P.; Willis, R.E.; Pressley, T.A.; Perez-Zoghbi, J.F. Ca2+ oscillations, Ca2+ sensitization, and contraction activated by protein kinase C in small airway smooth muscle. J. Gen. Physiol. 2013, 141, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.E.; Santana, L.F. A Ca2+- and PKC-driven regulatory network in airway smooth muscle. J. Gen. Physiol. 2013, 141, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Kume, H.; Oguma, T.; Shigemori, W.; Tohda, Y.; Ogawa, E.; Nakano, Y. Involvement of Ca2+ signaling in the synergistic effects between muscarinic receptor antagonists and β2-adrenoceptor agonists in airway smooth muscle. Int. J. Mol. Sci. 2016, 17, 1590. [Google Scholar] [CrossRef] [PubMed]
- The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2017. Available online: http://goldcopd.org (accessed on 20 December 2017).
- Dale, P.R.; Cernecka, H.; Schmidt, M.; Dowling, M.R.; Charlton, S.J.; Pieper, M.P.; Michel, M.C. The pharmacological rationale for combining muscarinic receptor antagonists and β-adrenoceptor agonists in the treatment of airway and bladder disease. Curr. Opin. Pharmacol. 2014, 16, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedzicha, J.A.; Decramer, M.; Ficker, J.H.; Niewoehner, D.E.; Sandstrom, T.; Taylor, A.F.; D’Andrea, P.; Arrasate, C.; Chen, H.; Banerji, D. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): A randomised, double-blind, parallel-group study. Lancet Respir. Med. 2013, 1, 199–209. [Google Scholar] [CrossRef]
- Decramer, M.; Anzueto, A.; Kerwin, E.; Kaelin, T.; Richard, N.; Crater, G.; Tabberer, M.; Harris, S.; Church, A. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: Results from two multicentre, blinded, randomised controlled trials. Lancet Respir. Med. 2014, 2, 472–486. [Google Scholar] [CrossRef]
- Buhl, R.; Maltais, F.; Abrahams, R.; Bjermer, L.; Derom, E.; Ferguson, G.; Flezar, M.; Hebert, J.; McGarvey, L.; Pizzichini, E.; et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur. Respir. J. 2015, 45, 969–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conn, P.J.; Christopoulos, A.; Lindsley, C.W. Allosteric modulators of GPCRs: A novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009, 8, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T.P. ‘7TM receptor allostery: Putting numbers to shapeshifting proteins. Trends Pharmacol. Sci. 2009, 30, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T.; Christopoulos, A. Signalling bias in new drug discovery: Detection, quantification and therapeutic impact. Nat. Rev. Drug Discov. 2013, 12, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Page, C.P.; Rogliani, P.; Facciolo, F.; Gavaldà, A.; Matera, M.G. Pharmacological characterization of the interaction between aclidinium bromide and formoterol fumarate on human isolated bronchi. Eur. J. Pharmacol. 2014, 745, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oguma, T.; Kume, H.; Ito, S.; Takeda, N.; Honjo, H.; Kodama, I.; Shimokata, K.; Kamiya, K. Involvement of reduced sensitivity to Ca2+ in β-adrenergic action on airway smooth muscle. Clin. Exp. Allergy 2006, 36, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kume, H.; Takeda, N.; Oguma, T.; Ito, S.; Kondo, M.; Ito, Y.; Shimokata, K. Sphingosine 1-phosphate causes airway hyper-reactivity by Rho-mediated myosin phosphatase inactivation. J. Pharmacol. Exp. Ther. 2007, 320, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Takai, A.; Tokuno, H.; Tomita, T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature 1989, 341, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Hall, I.P.; Washabau, R.J.; Takagi, K.; Kotlikoff, M.I. β-Adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J. Clin. Investig. 1994, 93, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Kotlikoff, M.I. Muscarinic inhibition of single KCa channels in smooth muscle cells by a pertussis-sensitive G protein. Am. J. Physiol. 1991, 261, C1204–C1209. [Google Scholar] [CrossRef] [PubMed]
- Sarria, B.; Naline, E.; Zhang, Y.; Cortijo, J.; Molimard, M.; Moreau, J.; Therond, P.; Advenier, C.; Morcillo, E.J. Muscarinic M2 receptors in acetylcholine-isoproterenol functional antagonism in human isolated bronchus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L1125–L1132. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Griffin, M.T.; Shehnaz, D.; Taketo, M.M.; Ehlert, F.J. Increased relaxant action of forskolin and isoproterenol against muscarinic agonist-induced contractions in smooth muscle from M2 receptor knockout mice. J. Pharmacol. Exp. Ther. 2003, 305, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Semenov, I.; Wang, B.; Herlihy, J.T.; Brenner, R. BK channel β1 subunits regulate airway contraction secondary to M2 muscarinic acetylcholine receptor mediated depolarization. J. Physiol. 2011, 589, 1803–1817. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Allosteric theory: Taking therapeutic advantage of the malleable nature of GPCRs. Curr. Neuropharmacol. 2007, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Melancon, B.J.; Hopkins, C.R.; Wood, M.R.; Emmitte, K.A.; Niswender, C.M.; Christopoulos, A.; Conn, P.J.; Lindsley, C.W. Allosteric modulation of seven transmembrane spanning receptors: Theory, practice, and opportunities for central nervous system drug discovery. J. Med. Chem. 2012, 55, 1445–1464. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Sharafkhaneh, A.; Barber, R.; Dickey, B.F. β-agonist intrinsic efficacy: Measurement and clinical significance. Am. J. Respir. Crit. Care Med. 2002, 165, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Kume, H. Clinical use of β2-adrenergic receptor agonists based on their intrinsic efficacy. Allergol. Int. 2005, 54, 89–97. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Puxeddu, E.; Ora, J.; Facciolo, F.; Rogliani, P.; Matera, M.G. Pharmacological characterisation of the interaction between glycopyrronium bromide and indacaterol fumarate in human isolated bronchi, small airways and bronchial epithelial cells. Respir. Res. 2016, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Takagi, K. Inhibitory effects of Gs on desensitization of β-adrenergic receptors in tracheal smooth muscle. Am. J. Physiol. 1997, 273, L556–L564. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Takagi, K. Inhibition of β-adrenergic desensitization by KCa channels in human trachealis. Am. J. Respir. Crit. Care Med. 1999, 159, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar] [PubMed]
- Goldoni, M.; Johansson, C. A mathematical approach to study combined effects of toxicants in vitro: Evaluation of the bliss independence criterion and the loewe additivity model. Toxicol. In Vitro 2007, 21, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Takagi, K.; Satake, T.; Tokuno, H.; Tomita, T. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. J. Physiol. 1990, 424, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, V.A.; Hirst, S.J.; Twort, C.H.; Ward, J.P. Potassium currents in human freshly isolated bronchial smooth muscle cells. Br. J. Pharmacol. 1995, 115, 1117–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikenouchi, T.; Kume, H.; Oguma, T.; Makino, Y.; Shiraki, A.; Ito, Y.; Shimokata, K. Role of Ca2+ mobilization in desensitization of β-adrenoceptors by platelet-derived growth factor in airway smooth muscle. Eur. J. Pharmacol. 2008, 591, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Girodet, P.O.; Dournes, G.; Thumerel, M.; Begueret, H.; Dos Santos, P.; Ozier, A.; Dupin, I.; Trian, T.; Montaudon, M.; Laurent, F.; et al. Calcium channel blocker reduces airway remodeling in severe asthma: A proof-of-concept study. Am. J. Respir. Crit. Care Med. 2015, 191, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Kume, H. Research and development for anti-asthmatic agents with a focus on phenotype changing by Ca2+ signaling in airway smooth muscle cells. In Frontiers in Clinical Drug Research—Anti Allergy Agents; Rahman, A.U., Ed.; Bentham: Sharjah, UAE, 2018; Volume 3, pp. 116–181. [Google Scholar]









| Muscarinic Receptor Antagonists | EC50 (nM) | Emax (%) | ||||
|---|---|---|---|---|---|---|
| MCh | MCh | |||||
| 1 μM (n = 6) | 10 μM (n = 8) | 1 μM (n = 6) | 10 μM (n = 8) | |||
| Mean ± S.D. | 95% CI | Mean ± S.D. | 95% CI | Mean ± S.D. | Mean ± S.D. | |
| Tiotropium | 3.3 ± 0.5 | 2.78–3.82 | 12.5 ± 3.2 * | 9.82–15.18 | 100 | 100 |
| Atropine | 5.7 ± 1.1 | 4.55–6.86 | 17.5 ± 5.9 * | 12.57–22.43 | 100 | 100 |
| Glycopyrronium | 8.2 ± 1.1 | 7.05–9.36 | 19.2 ± 7.1 * | 13.26–25.14 | 100 | 100 |
| β2-Adrenergic Receptor Agonists | EC50 (nM) | |||
| MCh | ||||
| 1 μM (n = 6) | 10 μM (n = 8) | |||
| Mean ± S.D. | 95% CI | Mean ± S.D. | 95% CI | |
| Procaterol | 1.9 ± 0.6 | 1.17–2.43 | 8.3 ± 2.6 * | 6.13–10.47 |
| Salbutamol | 18.6 ± 6.3 | 11.69–25.21 | 296.4 ± 90.6 ** | 228.21–335.59 |
| Formoterol | 0.7 ± 0.3 | 0.39–1.02 | 2.1 ± 0.3 ** | 1.85–2.35 |
| Isoproterenol | 308.1 ± 68.6 | 236.08–380.12 | 1736.4 ± 98.2 ** | 1654.48–1818.52 |
| β2-Adrenergic Receptor Agonists | Emax (%) | |||
| MCh | ||||
| 1 μM (n = 6) | 10 μM (n = 8) | |||
| Mean ± S.D. | 95% CI | Mean ± S.D. | 95% CI | |
| Procaterol | 97.6 ± 2.2 | 95.40–99.81 | 70.2 ± 9.3 ** | 62.42–77.98 |
| Salbutamol | 78.6 ± 8.4 | 69.78–87.42 | 51.0 ± 6.4 ** | 45.65–56.35 |
| Formoterol | 98.1 ± 1.6 | 96.42–99.78 | 71.0 ± 8.1 ** | 64.23–77.77 |
| Isoproterenol | 100 | 100 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kume, H.; Nishiyama, O.; Isoya, T.; Higashimoto, Y.; Tohda, Y.; Noda, Y. Involvement of Allosteric Effect and KCa Channels in Crosstalk between β2-Adrenergic and Muscarinic M2 Receptors in Airway Smooth Muscle. Int. J. Mol. Sci. 2018, 19, 1999. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19071999
Kume H, Nishiyama O, Isoya T, Higashimoto Y, Tohda Y, Noda Y. Involvement of Allosteric Effect and KCa Channels in Crosstalk between β2-Adrenergic and Muscarinic M2 Receptors in Airway Smooth Muscle. International Journal of Molecular Sciences. 2018; 19(7):1999. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19071999
Chicago/Turabian StyleKume, Hiroaki, Osamu Nishiyama, Takaaki Isoya, Yuji Higashimoto, Yuji Tohda, and Yukihiro Noda. 2018. "Involvement of Allosteric Effect and KCa Channels in Crosstalk between β2-Adrenergic and Muscarinic M2 Receptors in Airway Smooth Muscle" International Journal of Molecular Sciences 19, no. 7: 1999. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19071999