Cellular Sources and Regional Variations in the Expression of the Neuroinflammatory Marker Translocator Protein (TSPO) in the Normal Brain
Abstract
:1. Introduction
2. Results
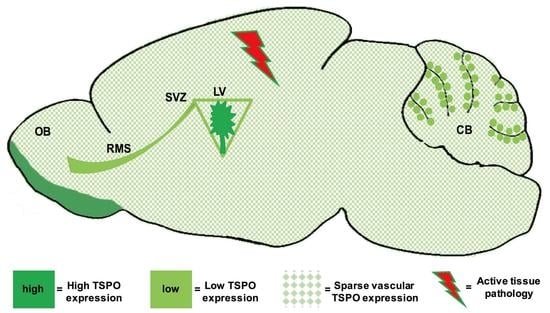
2.1. Regional Distribution
2.2. Cellular Distribution
3. Discussion
4. Materials and Methods
4.1. Animals and Tissue Preparation
4.2. Immunohistochemistry/Immunofluorescence
4.3. Microscopy
4.4. Semi-Quantification of TSPO Intensity and Colocalization
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| TSPO | Translocator protein |
| WT | Wild-type |
| BSA | Bovine serum albumin |
| DAB | 3,3′-diaminobenzidine |
| DAPI | 4’,6-diamidino-2-phenylindole |
| RMS | Rostral migratory stream |
| SVZ | Subventricular zone |
| NSC | Neural stem cells |
| PET | Positron emission tomography |
| ML | Molecular layer |
| PCL | Purkinje cell layer |
| GCL | Granule cell layer |
| DCN | Deep cerebellar nuclei |
References
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18kda): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Middleton, R.J.; Hatty, C.R.; Kam, W.W.; Chan, R.; Pham, T.; Harrison-Brown, M.; Dodson, E.; Veale, K.; Banati, R.B. The 18 kda translocator protein, microglia and neuroinflammation. Brain Pathol. 2014, 24, 631–653. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Middleton, R.J.; Chan, R.; Hatty, C.R.; Kam, W.W.; Quin, C.; Graeber, M.B.; Parmar, A.; Zahra, D.; Callaghan, P.; et al. Positron emission tomography and functional characterization of a complete pbr/tspo knockout. Nat. Commun. 2014, 5, 5452. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Zweckstetter, M.; Banati, R.B. Lost in translocation: The functions of the 18-kd translocator protein. Trends Endocrinol. Metab. 2015, 26, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Middleton, R.J.; Kam, W.W.; Chin, D.Y.; Hatty, C.R.; Chan, R.H.; Banati, R.B. Functional gains in energy and cell metabolism after tspo gene insertion. Cell Cycle 2017, 16, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B. Visualising microglial activation in vivo. GLIA 2002, 40, 206–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brendel, M.; Kleinberger, G.; Probst, F.; Jaworska, A.; Overhoff, F.; Blume, T.; Albert, N.L.; Carlsen, J.; Lindner, S.; Gildehaus, F.J.; et al. Increase of trem2 during aging of an alzheimer’s disease mouse model is paralleled by microglial activation and amyloidosis. Front. Aging Neurosci. 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Dedeurwaerdere, S.; Callaghan, P.D.; Pham, T.; Rahardjo, G.L.; Amhaoul, H.; Berghofer, P.; Quinlivan, M.; Mattner, F.; Loc’h, C.; Katsifis, A.; et al. Pet imaging of brain inflammation during early epileptogenesis in a rat model of temporal lobe epilepsy. EJNMMI Res. 2012, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, S.; Anzilotti, S.; Coda, A.R.; Gramanzini, M.; Greco, A.; Panico, M.; Vinciguerra, A.; Zannetti, A.; Vicidomini, C.; Dolle, F.; et al. Imaging of brain tspo expression in a mouse model of amyotrophic lateral sclerosis with (18)f-dpa-714 and micro-pet/ct. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Maeda, J.; Sawada, M.; Ono, M.; Okauchi, T.; Inaji, M.; Zhang, M.R.; Suzuki, K.; Ando, K.; Staufenbiel, M.; et al. Imaging of peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of alzheimer’s and other cns pathologies. J. Neurosci. 2008, 28, 12255–12267. [Google Scholar] [CrossRef] [PubMed]
- Li, H.D.; Li, M.; Shi, E.; Jin, W.N.; Wood, K.; Gonzales, R.; Liu, Q. A translocator protein 18 kda agonist protects against cerebral ischemia/reperfusion injury. J. Neuroinflamm. 2017, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, N.; Tang, S.P.; Ashworth, S.; Coello, C.; Plisson, C.; Passchier, J.; Selvaraj, V.; Tyacke, R.J.; Nutt, D.J.; Sastre, M. In vivo imaging of microglial activation by positron emission tomography with [(11)c]pbr28 in the 5xfad model of alzheimer’s disease. Glia 2016, 64, 993–1006. [Google Scholar] [PubMed]
- Pulagam, K.R.; Colas, L.; Padro, D.; Plaza-Garcia, S.; Gomez-Vallejo, V.; Higuchi, M.; Llop, J.; Martin, A. Evaluation of the novel tspo radiotracer [(18)f] vuiis1008 in a preclinical model of cerebral ischemia in rats. EJNMMI Res. 2017, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Fujinaga, M.; Mori, W.; Zhang, Y.; Wakizaka, H.; Nengaki, N.; Xie, L.; Hatori, A.; Zhang, M.R. In vivo monitoring for regional changes of metabotropic glutamate receptor subtype 1 (mglur1) in pilocarpine-induced epileptic rat brain by small-animal pet. Sci. Rep. 2017, 7, 14945. [Google Scholar] [CrossRef] [PubMed]
- Haarman, B.C.; Riemersma-Van der Lek, R.F.; de Groot, J.C.; Ruhe, H.G.; Klein, H.C.; Zandstra, T.E.; Burger, H.; Schoevers, R.A.; de Vries, E.F.; Drexhage, H.A.; et al. Neuroinflammation in bipolar disorder—A [(11)c]-(r)-pk11195 positron emission tomography study. Brain Behav. Immun. 2014, 40, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Hinz, R.; Conen, S.; Gregory, C.J.; Matthews, J.C.; Anton-Rodriguez, J.M.; Gerhard, A.; Talbot, P.S. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: A positron emission tomography study. Biol. Psychiatr. 2018, 83, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sagar, A.P.; Kéri, S. Translocator protein (18 kda tspo) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2018, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Notter, T.; Coughlin, J.M.; Gschwind, T.; Weber-Stadlbauer, U.; Wang, Y.; Kassiou, M.; Vernon, A.C.; Benke, D.; Pomper, M.G.; Sawa, A.; et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol. Psychiatr. 2018, 23, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatr. 2015, 72, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, M.A.; Zalesky, A.; O’Keefe, G.; Laskaris, L.; Baune, B.T.; Weickert, C.S.; Olver, J.; McGorry, P.D.; Amminger, G.P.; Nelson, B.; et al. Pet imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl. Psychiatr. 2017, 7, e1225. [Google Scholar] [CrossRef] [PubMed]
- Filiou, M.D.; Arefin, A.S.; Moscato, P.; Graeber, M.B. ‘Neuroinflammation’ differs categorically from inflammation: Transcriptomes of alzheimer’s disease, parkinson’s disease, schizophrenia and inflammatory diseases compared. Neurogenetics 2014, 15, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Bolger, G.T.; Mezey, E.; Cott, J.; Weissman, B.A.; Paul, S.M.; Skolnick, P. Differential regulation of ‘central’ and ‘peripheral’ benzodiazepine binding sites in the rat olfactory bulb. Euro. J. Pharmacol. 1984, 105, 143–148. [Google Scholar] [CrossRef]
- Anholt, R.R.; Murphy, K.M.; Mack, G.E.; Snyder, S.H. Peripheral-type benzodiazepine receptors in the central nervous system: Localization to olfactory nerves. J. Neurosci. 1984, 4, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Benavides, J.; Quarteronet, D.; Imbault, F.; Malgouris, C.; Uzan, A.; Renault, C.; Dubroeucq, M.C.; Gueremy, C.; Le Fur, G. Labelling of “peripheral-type” benzodiazepine binding sites in the rat brain by using [3h]pk 11195, an isoquinoline carboxamide derivative: Kinetic studies and autoradiographic localization. J. Neurochem. 1983, 41, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.T.; Schober, D.A.; Smalstig, E.B.; Mincy, R.E.; Gehlert, D.R.; Clemens, J.A. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J. Neurosci. 1995, 15, 5263–5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, J.G.; Mohler, H.; Haefely, W. Benzodiazepine binding sites: Receptors or acceptors? Trends Pharmacol. Sci. 1982, 3, 233–235. [Google Scholar] [CrossRef]
- Doble, A.; Malgouris, C.; Daniel, M.; Daniel, N.; Imbault, F.; Basbaum, A.; Uzan, A.; Gueremy, C.; Le Fur, G. Labelling of peripheral-type benzodiazepine binding sites in human brain with [3h]pk 11195: Anatomical and subcellular distribution. Brain Res. Bull. 1987, 18, 49–61. [Google Scholar] [CrossRef]
- Banati, R.B.; Newcombe, J.; Gunn, R.N.; Cagnin, A.; Turkheimer, F.; Heppner, F.; Price, G.; Wegner, F.; Giovannoni, G.; Miller, D.H.; et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: Quantitative in vivo imaging of microglia as a measure of disease activity. Brain 2000, 123, 2321–2337. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Myers, R.; Kreutzberg, G.W. Pk (‘peripheral benzodiazepine’)—binding sites in the cns indicate early and discrete brain lesions: Microautoradiographic detection of [3h]pk 11195 binding to activated microglia. J. Neurocytol. 1997, 26, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Turkheimer, F.E.; Edison, P.; Pavese, N.; Roncaroli, F.; Anderson, A.N.; Hammers, A.; Gerhard, A.; Hinz, R.; Tai, Y.F.; Brooks, D.J. Reference and target region modeling of [11c]-(r)-pk11195 brain studies. J. Nucl. Med. 2007, 48, 158–167. [Google Scholar] [PubMed]
- Rizzo, G.; Veronese, M.; Tonietto, M.; Bodini, B.; Stankoff, B.; Wimberley, C.; Lavisse, S.; Bottlaender, M.; Bloomfield, P.S.; Howes, O.; et al. Generalization of endothelial modelling of tspo pet imaging: Considerations on tracer affinities. J. Cereb. Blood Flow. Metab. 2017, 271678X17742004. [Google Scholar] [CrossRef] [PubMed]
- Wimberley, C.; Lavisse, S.; Brulon, V.; Peyronneau, M.A.; Leroy, C.; Bodini, B.; Remy, P.; Stankoff, B.; Buvat, I.; Bottlaender, M. Impact of endothelial translocator protein on the quantification of (18)f-dpa-714. J. Nucl. Med. 2018, 59, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Veronese, M.; Tonietto, M.; Zanotti-Fregonara, P.; Turkheimer, F.E.; Bertoldo, A. Kinetic modeling without accounting for the vascular component impairs the quantification of [(11)c]pbr28 brain pet data. J. Cereb. Blood Flow Metab. 2014, 34, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Veronese, M.; Reis Marques, T.; Bloomfield, P.S.; Rizzo, G.; Singh, N.; Jones, D.; Agushi, E.; Mosses, D.; Bertoldo, A.; Howes, O.; et al. Kinetic modelling of [(11)c]pbr28 for 18 kda translocator protein pet data: A validation study of vascular modelling in the brain using xbd173 and tissue analysis. J. Cereb. Blood Flow Metab. 2017, 271678X17712388. [Google Scholar]
- Tomasi, G.; Edison, P.; Bertoldo, A.; Roncaroli, F.; Singh, P.; Gerhard, A.; Cobelli, C.; Brooks, D.J.; Turkheimer, F.E. Novel reference region model reveals increased microglial and reduced vascular binding of 11c-(r)-pk11195 in patients with alzheimer’s disease. J. Nucl. Med. 2008, 49, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Roncaroli, F.; Su, Z.; Herholz, K.; Gerhard, A.; Turkheimer, F.E. Tspo expression in brain tumours: Is tspo a target for brain tumour imaging? Clin. Transl. Imaging 2016, 4, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Fonseca, E.S.; Oloris, S.C.; Matsuzaki, P.; Otake, A.H.; Leite, K.R.; Massoco, C.O.; Dagli, M.L.; Palermo-Neto, J. Effects of peripheral-type benzodiazepine receptor ligands on ehrlich tumor cell proliferation. Euro. J. Pharmacol. 2006, 550, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, M.; Fertikh, D.; Culty, M.; Li, H.; Vidic, B.; Papadopoulos, V. Peripheral-type benzodiazepine receptor (pbr) in human breast cancer: Correlation of breast cancer cell aggressive phenotype with pbr expression, nuclear localization, and pbr-mediated cell proliferation and nuclear transport of cholesterol. Cancer Res. 1999, 59, 831–842. [Google Scholar] [PubMed]
- Brown, R.C.; Degenhardt, B.; Kotoula, M.; Papadopoulous, V. Location-dependent role of the human glioma cell peripheral-type benzodiazepine receptor in proliferation and steroid biosynthesis. Cancer Lett. 2000, 156, 125–132. [Google Scholar] [CrossRef]
- Cosenza-Nashat, M.; Zhao, M.L.; Suh, H.S.; Morgan, J.; Natividad, R.; Morgello, S.; Lee, S.C. Expression of the translocator protein of 18 kda by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol. 2009, 35, 306–328. [Google Scholar] [CrossRef] [PubMed]
- Nava Catorce, M.; Acero, G.; Pedraza-Chaverri, J.; Fragoso, G.; Govezensky, T.; Gevorkian, G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in c57bl/6j mice. J. Neuroimmunol. 2016, 297, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Roncaroli, F.; Durrenberger, P.F.; Coope, D.J.; Karabatsou, K.; Hinz, R.; Thompson, G.; Turkheimer, F.E.; Janczar, K.; Plessis, D.D.; et al. The 18-kda mitochondrial translocator protein in human gliomas: An11c-(r)pk11195 pet imaging and neuropathology study. J. Nucl. Med. 2015, 56, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Notter, T.; Coughlin, J.M.; Sawa, A.; Meyer, U. Reconceptualization of translocator protein as a biomarker of neuroinflammation in psychiatry. Mol. Psychiatr. 2018, 23, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Little, P.; Arnberg, F.; Haggkvist, J.; Mulder, J.; Halldin, C.; Gulyas, B.; Holmin, S. Acute neuroinflammation in a clinically relevant focal cortical ischemic stroke model in rat: Longitudinal positron emission tomography and immunofluorescent tracking. Brain Struct. Func. 2016, 221, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavisse, S.; Guillermier, M.; Herard, A.S.; Petit, F.; Delahaye, M.; Van Camp, N.; Ben Haim, L.; Lebon, V.; Remy, P.; Dolle, F.; et al. Reactive astrocytes overexpress tspo and are detected by tspo positron emission tomography imaging. J. Neurosci. 2012, 32, 10809–10818. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Fan, J.; Papadopoulos, V. Translocator protein (tspo) gene promoter-driven green fluorescent protein synthesis in transgenic mice: An in vivo model to study tspo transcription. Cell. Tissue Res. 2012, 350, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhai, K.; Xue, Y.; Yang, J.; Yang, Q.; Fu, Y.; Hu, Y.; Liu, F.; Wang, W.; Cui, L.; et al. Global deletion of tspo does not affect the viability and gene expression profile. PLoS ONE 2016, 11, e0167307. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.N.; Morohaku, K.; Manna, P.R.; Pelton, S.H.; Butler, W.R.; Stocco, D.M.; Selvaraj, V. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J. Biol. Chem. 2014, 289, 27444–27454. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Muzik, O.; Shandal, V.; Chugani, D.; Chakraborty, P.; Chugani, H.T. Evaluation of age-related changes in translocator protein (tspo) in human brain using 11c-[r]-pk11195 pet. J. Neuroinflamm. 2012, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Winkeler, A.; Boisgard, R.; Awde, A.R.; Dubois, A.; Theze, B.; Zheng, J.; Ciobanu, L.; Dolle, F.; Viel, T.; Jacobs, A.H.; et al. The translocator protein ligand [(1)(8)f]dpa-714 images glioma and activated microglia in vivo. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Mattner, F.; Staykova, M.; Berghofer, P.; Wong, H.J.; Fordham, S.; Callaghan, P.; Jackson, T.; Pham, T.; Gregoire, M.C.; Zahra, D.; et al. Central nervous system expression and pet imaging of the translocator protein in relapsing-remitting experimental autoimmune encephalomyelitis. J. Nucl. Med. 2013, 54, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Langlet, F.; Mullier, A.; Bouret, S.G.; Prevot, V.; Dehouck, B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J. Comp. Neurol. 2013, 521, 3389–3405. [Google Scholar] [CrossRef] [PubMed]
- Kyrousi, C.; Lygerou, Z.; Taraviras, S. How a radial glial cell decides to become a multiciliated ependymal cell. Glia 2017, 65, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Götz, M. Glial control of neurogenesis. Curr. Opin. Neurobiol. 2017, 47, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Engelhardt, B. The circumventricular organs participate in the immunopathogenesis of experimental autoimmune encephalomyelitis. Cerebrospinal Fluid Res. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Korr, H.; Schultze, B.; Maurer, W. Autoradiographic investigations of glial proliferation in the brain of adult mice. I. The DNA synthesis phase of neuroglia and endothelial cells. J. Comp. Neurol. 1973, 150, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Gatliff, J.; Campanella, M. The 18 kda translocator protein (tspo): A new perspective in mitochondrial biology. Curr. Mol. Med. 2012, 12, 356–368. [Google Scholar] [PubMed]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [PubMed]
- Misgeld, T.; Schwarz, T.L. Mitostasis in neurons: Maintaining mitochondria in an extended cellular architecture. Neuron 2017, 96, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates; Elsevier Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]






| Brain Region | Endothelial Cells (CD31+) | Microglia (CD11b+) | Astrocytes/NSC (GFAP+) | NSC (Nestin+) | Pericytes (PDGFRβ+) | Purkinje Cells (Calbindin+) | Oligodendrocytes (MBP+) | Mature Neurons (NeuN+) | Ependyma |
|---|---|---|---|---|---|---|---|---|---|
| Olfactory bulb | ++ | − | ++ | ++ | + | − | − | − | − |
| SVZ | ++ | − | ++ | ++ | + | − | − | − | − |
| Cortex | ++ | − | − | − | + | − | − | − | − |
| Choroid plexus/ventricular system | − | − | − | − | − | − | − | − | ++ |
| Hippocampus | ++ | − | + | + | + | − | − | − | − |
| Striatum/thalamus | ++ | − | − | − | + | − | − | − | − |
| Cerebellum/brainstem | ++ | − | − | − | + | ++ | − | − | − |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betlazar, C.; Harrison-Brown, M.; Middleton, R.J.; Banati, R.; Liu, G.-J. Cellular Sources and Regional Variations in the Expression of the Neuroinflammatory Marker Translocator Protein (TSPO) in the Normal Brain. Int. J. Mol. Sci. 2018, 19, 2707. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092707
Betlazar C, Harrison-Brown M, Middleton RJ, Banati R, Liu G-J. Cellular Sources and Regional Variations in the Expression of the Neuroinflammatory Marker Translocator Protein (TSPO) in the Normal Brain. International Journal of Molecular Sciences. 2018; 19(9):2707. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092707
Chicago/Turabian StyleBetlazar, Calina, Meredith Harrison-Brown, Ryan J. Middleton, Richard Banati, and Guo-Jun Liu. 2018. "Cellular Sources and Regional Variations in the Expression of the Neuroinflammatory Marker Translocator Protein (TSPO) in the Normal Brain" International Journal of Molecular Sciences 19, no. 9: 2707. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092707