1. Introduction
Tacrolimus has been widely used as an immunosuppressive drug for prophylaxis of graft-versus-host disease (GVHD) after allogenic hematopoietic stem cell transplantation (HSCT) [
1,
2,
3,
4,
5,
6]. GVHD remains the major cause of morbidity and mortality in recipients after HSCT; therefore, prevention of severe GVHD is crucial for the successful treatment of GVHD [
7]. Given the narrow therapeutic range and the large inter- and intra-individual variabilities in the pharmacokinetics of tacrolimus [
8], its blood concentration should be maintained at adequate levels to prevent drug-related toxicities. Tacrolimus is administered intravenously in the early phase after HSCT, followed by switching to an oral formulation when tolerated by recipients.
Single nucleotide polymorphisms (SNPs) in the genes encoding drug-metabolizing enzymes are considered to affect the pharmacokinetics of tacrolimus. Cytochrome P450 3A4 (CYP3A4) and CYP3A5 contribute to inter-individual variability in the metabolism of tacrolimus. Moreover, CYP3A5 may dominate CYP3A4 in the metabolism of tacrolimus in individuals expressing the CYP3A5 enzyme [
9,
10,
11,
12,
13]. The most important SNP related to functional variation is
CYP3A5*3 (6986A > G, rs776746), which causes abnormal mRNA splicing, resulting in a non-functional CYP3A5 protein [
14,
15]. Racial differences in the frequencies of
CYP3A5 polymorphisms are well acknowledged. The frequencies of
CYP3A5*3/*3 have been reported to be 65%–73% in Asians, 87%–95% in Caucasians, and 27%–50% in the African-American population [
16]. The majority of previous reports on organ transplant recipients show a significant effect of
CYP3A5*3 on the pharmacokinetics of tacrolimus [
17]. In HSCT recipients, several studies have shown that the concentration/dose (C/D) ratio or trough-blood concentration of tacrolimus is higher in recipients with the
CYP3A5*3/*3 genotype than in those with the
CYP3A5*1 allele (
*1/*1 and
*1/*3 genotypes) and that the required daily dosage of tacrolimus is, thus, significantly reduced [
18,
19,
20,
21]. However, reports discussing the utility of information related to
CYP3A5 polymorphism at the time of continuous intravenous infusion [
18,
19,
20] and oral administration [
21] are limited. Therefore, the effect of
CYP3A5 polymorphism on the pharmacokinetics of tacrolimus when switching from continuous intravenous infusion to oral administration is unclear. In contrast, although there are many genetic variants of
CYP3A4, the majority of studies have failed to find an association between the
CYP3A4 genotype and tacrolimus pharmacokinetics [
10,
18,
20]. Similarly, many studies suggest that SNPs in the ATP-binding cassette subfamily B member 1 transporter (
ABCB1) gene do not influence tacrolimus pharmacokinetics, especially in Asian populations [
10,
18,
20,
22].
Genes located outside of the
CYP3A locus may also influence the CYP3A phenotype. Cytochrome P450 oxidoreductase (POR) has recently been recognized as a potential contributor to intra- and inter-individual variability and an influencer of CYP3A activity. POR is involved in electron transfer from NADPH to the microsomal CYP enzymes, including members of the CYP3A subfamily, enabling their activities [
23,
24]. Human POR is highly polymorphic, and the most common sequence variant
POR*28 (1508C > T; rs1057868) induces an amino acid substitution (Ala503Val) [
25]. This substitution influences the electron-binding moiety of POR. The
POR*28 SNP varies in frequency: 36% in Chinese-Americans, 26.4% in Caucasians, 19.1% in African-Americans, and 31% in Mexicans [
25]. Those with the
CYP3A5*1 allele carrying one or two
POR*28 alleles (
*1/*28 and
*28/*28 genotypes) have lower tacrolimus C/D ratios and higher tacrolimus dose requirements than those with the
CYP3A5*1 allele without
POR*28 (
*1/*1 genotype) among kidney transplant recipients [
26]. However, it is still unclear whether the
POR*28 polymorphism affects the pharmacokinetics of tacrolimus in HSCT recipients, as there are no reports describing this in HSCT.
When performing HSCT, azole antifungal agents such as fluconazole (FLCZ) and itraconazole (ITCZ) are most often used for the prevention or treatment of fungal infection [
27,
28]. The azole antifungal agents primarily inhibit the CYP3A4 enzyme [
29,
30,
31]. Therefore, avoiding drug–drug interactions between tacrolimus and azole antifungal agents is difficult in HSCT, and differences in
CYP3A5 genotype may affect the interactions between these drugs. In addition, voriconazole (VRCZ), another azole antifungal agent, is metabolized by CYP2C19 as well as by CYP3A [
32]. In particular, 15%–20% of Asians and 3%–5% of whites and blacks are estimated to be poor metabolizers of CYP2C19 [
33]. Imamura et al. [
34] reported that
CYP2C19 polymorphism is one of the key factors affecting the pharmacokinetics of tacrolimus in the concomitant administration of VRCZ in healthy Japanese volunteers. Therefore, the degree of drug interaction between tacrolimus and VRCZ may be influenced by
CYP2C19 and
CYP3A5 genotypes.
Accordingly, if information regarding CYP3A5, POR, and CYP2C19 polymorphisms can be obtained prior to administration of tacrolimus, it would enable the adjustment of the initial dosage of tacrolimus and a reduction in the risk of adverse reactions. However, the clinical usefulness of such genetic polymorphism information in HSCT recipients has not yet been fully elucidated. In the present study, we examined the effect of gene polymorphisms on the pharmacokinetics of tacrolimus during the early stage of continuous intravenous infusion and when switching from continuous intravenous infusion to oral administration in HSCT recipients. We focused on the CYP3A5, POR, and CYP2C19 polymorphisms and the interactions of tacrolimus with azole antifungal agents.
3. Discussion
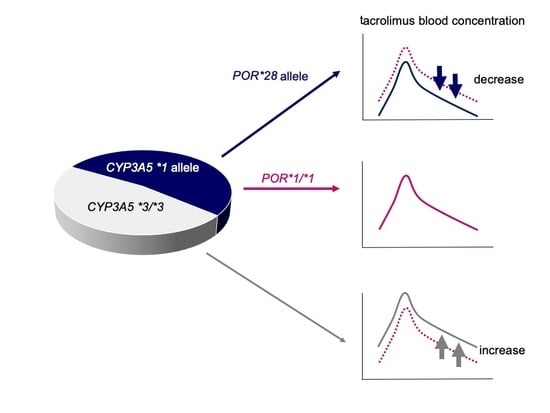
The main findings of this study are as follows: (1) HSCT recipients with the CYP3A5*1 allele, particularly those with at least one POR*28 allele, had a significantly reduced tacrolimus C/D ratio compared to that in HSCT recipients with POR*1/*1 during continuous intravenous infusion; (2) the CYP3A5*3/*3 genotype and the concomitant use of VRCZ are independent factors leading to an increased tacrolimus C/D ratio during switching the route of administration; and (3) conversion from intravenous to oral administration of tacrolimus at a ratio of 1:5 seemed appropriate in recipients carrying the CYP3A5*1 allele, while a lower conversion ratio, for instance 1:2–3, was appropriate in HSCT recipients with CYP3A5*3/*3.
There are several reports describing higher tacrolimus C/D ratios or trough-blood concentrations in HSCT recipients with
CYP3A5*3/*3 than in those with the
CYP3A5 *1 allele during continuous intravenous infusion of tacrolimus [
18,
19,
20]. Our results are consistent with those reported previously. To our knowledge, our study demonstrates for the first time that HSCT recipients with the
CYP3A5*1 allele, and those with at least one
POR*28 allele, exhibit significantly lower tacrolimus C/D ratios than HSCT recipients without a
POR*28 allele during continuous intravenous infusion (
Table 3). This finding is consistent with respect to findings in kidney [
26,
35,
36,
37,
38] and heart [
39] transplant recipients. Therefore, the
POR*28 allele is considered to enhance the metabolic activity of CYP3A5, rather than that of CYP3A4. In contrast, HSCT recipients with
CYP3A5*3/*3 carrying one or two
POR*28 alleles had significantly lower tacrolimus C/D ratios compared to those in recipients without
POR*28 during the first week following HSCT. However, no significant differences were observed after excluding the influence of concomitant use of azole antifungal agents (
Table 4). These results suggest that azole antifungal agents primarily inhibit the activity of CYP3A4 and that tacrolimus is mainly metabolized by CYP3A4 in subjects with
CYP3A5*3/*3. Zhang et al. [
40] reported similar results, in that the
POR*1/*28 genotype led to a significantly lower level of tacrolimus exposure than
POR28*1/*1 in
CYP3A5*1/*1 carriers. Interestingly, this phenomenon disappeared in
CYP3A5*3/*3 carriers identified among 71 healthy Chinese volunteers. Because the effect of the
POR polymorphism on the metabolism of tacrolimus via CYP3A5 has not yet been fully clarified in vitro, further research will be needed.
In this study, the relationship between acute GVHD and
CYP3A5 polymorphism within four weeks after HSCT was investigated. We observed that the cumulative incidence of grade 2–4 acute GVHD tended to be higher in individuals with the
CYP3A5*1 allele than in those with
CYP3A5*3/*3. Khaled et al. [
20] reported that eight recipients with
CYP3A5*1/*1 exhibited a significantly higher cumulative rate of grade 2–4 acute GVHD than recipients with
CYP3A5*1/*3 (
n = 40) and
CYP3A5*3/*3 (
n = 122) within 100 days after HSCT. Yamashita et al. [
21] reported that recipients with the
CYP3A5*1 allele (
n = 11) exhibited a significantly higher cumulative rate of grade 3–4 acute GVHD than those with
CYP3A5*3/*3 (
n = 13) within 100 days after HSCT. In this study, although there were three
CYP3A5*1/*1 recipients (data not shown), grade 3–4 acute GVHD occurred in only two recipients (data not shown). Thus, although a low C/D ratio or blood concentration of tacrolimus may be associated with the
CYP3A5*1 allele rather than with the
CYP3A5*3/*3 genotype, statistical analysis could not be performed due to the small number of cases; studies including a large number of subjects are therefore needed.
The influence of
CYP3A5 polymorphism on the tacrolimus (C/Dpo)/(C/Div) ratio when switching the drug administration route from continuous intravenous infusion to oral administration was significantly higher in those with
CYP3A5*3/*3 than in those with the
CYP3A5*1 allele (
Figure 2B). This result suggests that CYP3A5 is expressed in the liver and small intestine, and indeed, CYP3A5 has been shown to play a very important role in the small intestine [
9,
41,
42,
43,
44]. In this study, multiple regression analysis revealed that
CYP3A5*3/*3 was one of the independent factors contributing to a significant increase in (C/Dpo)/(C/Div). Moreover, the (C/Dpo)/(C/Div) of tacrolimus tended to be higher in recipients with
CYP3A5*3/*3 and those receiving azole antifungal agents than in those with the
CYP3A5*1 allele (
Figure 3). Yamashita et al. [
21] reported that among recipients undergoing concomitant use of azole antifungal agents, the trough concentration of tacrolimus was higher in recipients with
CYP3A5*3/*3 than in those with the
CYP3A5*1 allele, although the daily doses of once-daily modified-release tacrolimus formulations in recipients with
CYP3A5*3/*3 were significantly lower than in those with the
CYP3A5*1 allele. Azole antifungal agents have a stronger inhibitory effect on CYP3A4 activity than on CYP3A5 in the small intestine [
29,
31], and tacrolimus is metabolized by CYP3A4 in recipients expressing
CYP3A5*3/*3. As a result of CYP3A4 inhibition by azole antifungal agents in the small intestine, the (C/Dpo)/(C/Div) of tacrolimus tends to be higher in
CYP3A5*3/*3 recipients when azole antifungal agents are co-administered. Therefore, when switching the route of administration, it is important to consider the combination of the
CYP3A5 genotype and the concomitant use of azole antifungal agents in addition to the therapeutic drug monitoring of tacrolimus. Tacrolimus is often concomitantly administered with PPIs, giving rise to drug interaction issues. Interactions between tacrolimus and PPIs are affected by a combination of the CYP2C19 and CYP3A5 polymorphisms [
45]. In this study, concomitant administration of omeprazole or lansoprazole, both of which increase the C/D ratio of tacrolimus, did not significantly impact the increase in the (C/Dpo)/(C/Div) of tacrolimus. Since the number of cases included in this study was small, this may need to be confirmed in a larger number of cases.
In this study, the concomitant use of VRCZ was identified as another independent factor that significantly increased the (C/Dpo)/(C/Div) of tacrolimus. Imamura et al. [
34] reported that
CYP2C19 PMs and IMs achieve 4- and 2-fold higher VRCZ exposures (areas under the curve), respectively, than that achieved by EMs. Iwamoto et al. [
19] reported that the dose of tacrolimus in continuous intravenous infusion varies depending on the combination of
CYP3A5 and
CYP2C19 genotypes in HSCT recipients treated with VRCZ. In this study, as we analyzed only seven recipients with VRCZ use, we could not statistically analyze the effects of the combination of
CYP3A5 and
CYP2C19 genotypes on the (C/Dpo)/(C/Div) of tacrolimus (
Table 7). Both the two recipients with high (C/Dpo)/(C/Div) of tacrolimus and those with low (C/Dpo)/(C/Div) were of the
CYP3A5*3/*3 and
CYP2C19 IM genotypes. However, the trough plasma concentrations of VRCZ in the latter two recipients were near the lower limit of the recommended target trough value of >1–2 mcg/mL [
46]. These findings can be explained on the basis of in vitro human liver microsome experiments that demonstrated that the magnitude of the inhibition of tacrolimus metabolism by VRCZ is concentration dependent [
47]. Therefore, our results suggest the importance of not only the combination of the
CYP3A5 and
CYP2C19 genotypes, but also the plasma concentration of VRCZ.
According to the guidelines for GVHD from the Japanese Society for Hematopoietic Cell Transplantation, a 3- to 4-fold higher dosage is recommended when switching from continuous intravenous infusion to oral administration of tacrolimus with HSCT. We have previously reported that switching from intravenous to oral administration at a 1:5 ratio seems appropriate and that a lower conversion ratio, such as 1:3, is appropriate for patients taking oral ITCZ or VRCZ [
48]. However, these recommendations did not consider the genetic polymorphisms, especially of
CYP3A5, that affect the pharmacokinetics of tacrolimus. In this study, multiple regression analysis revealed the
CYP3A5*3/*3 genotype as an independent factor significantly increasing (C/Dpo)/(C/Div). Our result indicates that switching from intravenous to oral administration of tacrolimus at a ratio of 1:5 in recipients with the
CYP3A5*1 allele is seemingly appropriate, while a lower conversion ratio such as 1:2–3 may be suitable in recipients with
CYP3A5*3/*3 (
Figure 4). Moreover, the concomitant use of VRCZ is also a significant independent factor leading to increased tacrolimus (C/Dpo)/(C/Div), and large individual variation was observed in the (C/Dpo)/(C/Div) of tacrolimus. Therefore, conversion is recommended under close medical supervision, and readjustment of the tacrolimus dose should be done in consideration of its blood level.
This study had several limitations. First, this study had a small sample size of only 36 cases and was conducted at a single facility. Second, the initial dosage and the conversion ratio of tacrolimus between intravenous and oral administration were not standardized because this was an observational study. Regardless of the limitations described above, our results indicate that the CYP3A5, POR, and CYP2C19 polymorphisms are useful for the dosage adjustment of tacrolimus in association with estimation of the systemic pharmacokinetics of tacrolimus. It may be important to adjust the target level of tacrolimus both after the initial post-transplantation period on the basis of the CYP3A5 genotype in combination with the POR genotype and when switching the administration route based on the CYP3A5 genotype and combination with the CYP2C19 genotype in recipients receiving VRCZ.