The Effects of Poncirus fructus on Insulin Resistance and the Macrophage-Mediated Inflammatory Response in High Fat Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Results
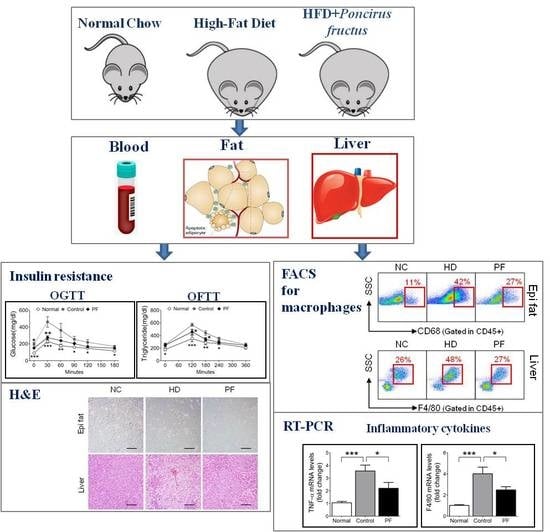
2.1. Effects of PF on Body Weight and Epididymal Fat and Liver Weight Changes
2.2. Effects of PF on Fat Accumulation on Epididymal Fat and Liver
2.3. Effects of PF on Insulin Resistance and Glucose Tolerance Test
2.4. Effects of PF on Lipid Metabolism and Fat Tolerance Test
2.5. Effects of PF on Adipose Tissue Macrophages (ATMs) and Liver Kupffer Cells
2.6. Effects of PF on Inflammatory Gene Expression
3. Discussion
4. Materials and Methods
4.1. Preparation of Poncirus fructus
4.2. Experimental Design and Animals
4.3. Oral Glucose Tolerance Test and Blood Analysis
4.4. Oral Fat Tolerance Test (OFTT)
4.5. RNA Isolation and Analysis of Gene Expression
4.6. Isolation of Stromal Vascular Cells (SVCs) and Liver Immune Cells
4.7. Fluorescence-Activated Cell Sorting (FACS) Analysis of Adipose Tissue Macrophages (ATMs) and Kupffer Cells
4.8. Histological Analyses of Adipose Tissue and Liver
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007, 56, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Thematic review series: Adipocyte biology. Adipocyte stress: The endoplasmic reticulum and metabolic disease. J. Lipid Res. 2007, 48, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Surmi, B.K.; Hasty, A.H. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008, 3, 545–556. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Sethi, J.K. Tnf-alpha and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. Tlr4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubler, M.J.; Kennedy, A.J. Role of lipids in the metabolism and activation of immune cells. J. Nutr. Biochem. 2016, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Seo, E.K.; Chung, S.J.; Shim, C.K. Prokinetic activity of an aqueous extract from dried immature fruit of poncirus trifoliata (l.) raf. J. Ethnopharmacol. 2005, 102, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-J.; Jun, J.-H.; Kim, T.-J.; Suh, D.-K.; Youn, D.-H.; Kim, T.-W. The relaxing effect of poncirus fructus and its flavonoid content on porcine coronary artery. Lab. Anim. Res. 2015, 31, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, E.K.; Shim, W.S. Phytotherapeutic effects of the fruits of poncirus trifoliata (l.) raf. On cancer, inflammation, and digestive dysfunction. Phytother. Res. 2018, 32, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.F.; Saravanakumar, K.; Wang, M.H.; Kim, H.Y. Antioxidant activity of ethanol extract of poncirus fructus immaturus. Res. J. Biotechnol. 2019, 14, 1–4. [Google Scholar]
- Jia, S.; Gao, Z.; Yan, S.; Chen, Y.; Sun, C.; Li, X.; Chen, K. Anti-obesity and hypoglycemic effects of poncirus trifoliata l. Extracts in high-fat diet c57bl/6 mice. Molecules 2016, 21, 453. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-T.; Seo, E.-K.; Chung, S.-J.; Shim, C.-K. Effect of an aqueous extract of dried immature fruit of poncirus trifoliata (l.) raf. On intestinal transit in rodents with experimental gastrointestinal motility dysfunctions. J. Ethnopharmacol. 2005, 102, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The epidemiology of obesity: A big picture. PharmacoEconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Parhofer, K.G. Interaction between glucose and lipid metabolism: More than diabetic dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Bharti, S.; Ojha, S.; Bhatia, J.; Kumar, N.; Ray, R.; Kumari, S.; Arya, D.S. Up-regulation of ppargamma, heat shock protein-27 and -72 by naringin attenuates insulin resistance, beta-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br. J. Nutr. 2011, 106, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Gao, D.-M.; Mohamed, S.; Chen, J.; Zhang, J.; Zhou, X.-Y.; Zhou, N.-J.; Xie, J.; Jiang, H. Naringin ameliorates metabolic syndrome by activating amp-activated protein kinase in mice fed a high-fat diet. Arch. Biochem. Biophys. 2012, 518, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Yun, S.I.; Kim, B.Y.; Jin, Q.; Woo, E.R.; Jeong, S.Y.; Chung, Y.S. Poncirin promotes osteoblast differentiation but inhibits adipocyte differentiation in mesenchymal stem cells. Eur. J. Pharmacol. 2011, 664, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Seo, E.K.; Lee, K.T. Inhibition of lps-induced inos, cox-2 and cytokines expression by poncirin through the nf-kappab inactivation in raw 264.7 macrophage cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ding, Q.; Muranaka, S.; Kigure, M.; Kojima, M.; Terada, M.; Kataoka, N.; Hagiwara, A.; Kromkhun, P.; Moritani, N.; et al. Correlation between body weight (epididymal fat) and permeation rate of serum leptin through the blood-brain barrier (bbb) in male rats aged 8 months. Exp. Anim. 2008, 57, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, G.; Yue, P.; Lin, X.; Chen, Z. Fatty liver and insulin resistance: Not always linked. Trans. Am. Clin. Clim. Assoc. 2008, 119, 217–223. [Google Scholar]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.S.; Back, H.; Seo, E.K.; Lee, H.T.; Shim, C.K. Long-term administration of an aqueous extract of dried, immature fruit of poncirus trifoliata (l.) raf. Suppresses body weight gain in rats. J. Ethnopharmacol. 2009, 126, 294–299. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Zhang, Y.L.; Hernandez-Ono, A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch. Med. Res. 2005, 36, 232–240. [Google Scholar] [CrossRef]
- Albalat, A.; Saera-Vila, A.; Capilla, E.; Gutiérrez, J.; Pérez-Sánchez, J.; Navarro, I. Insulin regulation of lipoprotein lipase (lpl) activity and expression in gilthead sea bream (sparus aurata). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 151–159. [Google Scholar] [CrossRef]
- Sparks, J.D.; Sparks, C.E.; Adeli, K. Selective hepatic insulin resistance, vldl overproduction, and hypertriglyceridemia. Arter. Thromb. Vasc. Biol. 2012, 32, 2104–2112. [Google Scholar] [CrossRef]
- Gerich, J.E. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 2002, 51, S117. [Google Scholar] [CrossRef]
- Dalmas, E.; Clement, K.; Guerre-Millo, M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011, 32, 307–314. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007, 117, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725. [Google Scholar] [CrossRef]
- Komohara, Y.; Fujiwara, Y.; Ohnishi, K.; Shiraishi, D.; Takeya, M. Contribution of macrophage polarization to metabolic diseases. J. Atheroscler. Thromb. 2016, 23, 10–17. [Google Scholar] [CrossRef]
- Wentworth, J.M.; Naselli, G.; Brown, W.A.; Doyle, L.; Phipson, B.; Smyth, G.K.; Wabitsch, M.; O’Brien, P.E.; Harrison, L.C. Pro-inflammatory cd11c+cd206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 2010, 59, 1648–1656. [Google Scholar] [CrossRef]
- Austyn, J.M.; Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981, 11, 805–815. [Google Scholar] [CrossRef]
- Kinoshita, M.; Uchida, T.; Sato, A.; Nakashima, M.; Nakashima, H.; Shono, S.; Habu, Y.; Miyazaki, H.; Hiroi, S.; Seki, S. Characterization of two f4/80-positive kupffer cell subsets by their function and phenotype in mice. J. Hepatol. 2010, 53, 903–910. [Google Scholar] [CrossRef]
- Suganami, T.; Nishida, J.; Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor alpha. Arter. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Seol, M.H.; Lee, B.-C. The Effects of Poncirus fructus on Insulin Resistance and the Macrophage-Mediated Inflammatory Response in High Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2019, 20, 2858. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20122858
Kim M, Seol MH, Lee B-C. The Effects of Poncirus fructus on Insulin Resistance and the Macrophage-Mediated Inflammatory Response in High Fat Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2019; 20(12):2858. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20122858
Chicago/Turabian StyleKim, Mia, Mi Hyeon Seol, and Byung-Cheol Lee. 2019. "The Effects of Poncirus fructus on Insulin Resistance and the Macrophage-Mediated Inflammatory Response in High Fat Diet-Induced Obese Mice" International Journal of Molecular Sciences 20, no. 12: 2858. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20122858