Fluorescence Quenching-Based Mechanism for Determination of Hypochlorite by Coumarin-Derived Sensors
Abstract
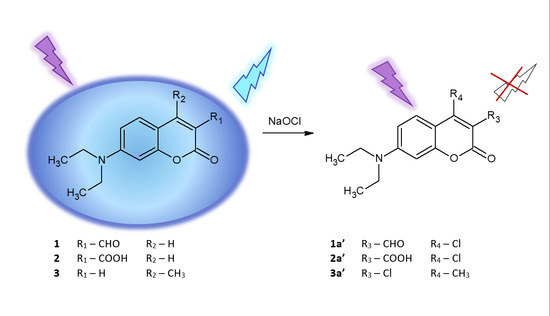
:1. Introduction
2. Results and Discussion
2.1. Determination of the Effect of Hypochlorite on the Emission Properties of the Probes
2.2. The pH Effects on the Hypochlorite Detection by Coumarin-Derived Probes
2.3. Mass Spectrometry Analysis of the Reaction Mixtures
2.4. Investigation into the Fluorescence of Probes in the Presence of Anti-Hypochlorite Agent Trolox
2.5. Assessment of Applicability of Probes 1–3 as Tools for Quantitative Determination of Hypochlorite
2.6. Isolation and Structural Characterisation of Chlorinated Derivative 2a′
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthesis of Coumarin Derivatives
3.3.1. Synthesis of 7-Diethylamino-3-Formylcoumarin 1
3.3.2. Synthesis of 7-Diethylaminocoumarin 3-Carboxylic Acid 2
3.3.3. Isolation of Chlorinated Derivative of 7-Diethylaminocoumarin 3-Carboxylic Acid 2a′
3.4. Fluorescence Assay
3.4.1. Determination of the Effect of Hypochlorite on the Emission Properties of the Probes
3.4.2. Investigation into the Fluorescence of Probes in the Presence of Anti-Hypochlorite Agent
3.5. Mass Spectrometry Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ICT | intramolecular charge transfer |
| PET | photO−induced electron transfer |
| FRET | fluorescence resonance energy transfer |
| ESIPT | excited state intramolecular proton transfer |
| ROS | reactive oxygen species |
| HCSe | boron-dipyrromethene-based turn-on fluorescent probe |
| ClO− | hypochlorite ion |
| PDA | photodiode array |
| ESI | electrospray ionisation |
References
- Song, Y.; Zhen, C.; Li, H. Advances in Coumarin-Derived Fluorescent Chemosensors for Metal Ions. Curr. Org. Chem. 2012, 16, 2690–2707. [Google Scholar] [CrossRef]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, R.; Jain, Y.; Kumari, M.; Agarwal, M. Recent trends in coumarin based neutral synthetic receptors for naked eye sensing of anions. Chem. Biol. Interface 2017, 7, 102–115. [Google Scholar]
- Wang, L.; Li, W.; Zhi, W.; Ye, D.; Zhang, W.; Ni, L. Rapid detection of hypochlorite by a coumarin-based hydrazide in aqueous solution and its application in live-cell imaging. Sens. Actuators B 2018, 255, 1112–1118. [Google Scholar] [CrossRef]
- Li, C.; Li, S.; Yang, Z. Development of a coumarin-furan conjugate as Zn2+ ratiometric fluorescent probe in ethanol-water system. Spectrochim. Acta A 2017, 174, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Wu, Y.; Wang, L.; Gong, A.; Hu, F.; Zhang, C. A fluorescent probe for hypochlorite based on the modulation of the unique rotation of the N–N single bond in acetohydrazide. Chem. Commun. 2015, 51, 10435–10438. [Google Scholar] [CrossRef]
- Jung, H.S.; Kwon, P.S.; Lee, J.W.; Kim, J.I.; Hong, C.S.; Kim, J.W.; Yan, S.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-Derived Cu2+-Selective Fluorescence Sensor: Synthesis, Mechanisms, and Applications in Living Cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterization and antimicrobial activity of a series of substituted coumarin-3-carboxylatosilver(I) complexes. Inorg. Chim. Acta 2006, 359, 3976–3984. [Google Scholar] [CrossRef] [Green Version]
- Creaven, B.S.; Egan, D.A.; Karcz, D.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterisation and antimicrobial activity of copper(II) and manganese(II) complexes of coumarin-6,7-dioxyacetic acid (cdoaH(2)) and 4-methylcoumarin-6,7-dioxyacetic acid (4-MecdoaH(2)): X-ray crystal structures of Cu(cdoa)(phen)(2)•8.8H2O and Cu(4-Mecdoa)(phen)(2)•13H(2)O (phen = 1,10-phenanthroline). J. Inorg. Biochem. 2007, 101, 1108–1119. [Google Scholar] [CrossRef]
- Creaven, B.S.; Devereux, M.; Karcz, D.; Kellett, A.; McCann, M.; Noble, A.; Walsh, M. Copper(II) complexes of coumarin-derived Schiff bases and their anti-Candida activity. J. Inorg. Biochem. 2009, 103, 1196–1203. [Google Scholar] [CrossRef]
- Creaven, B.S.; Czegledi, E.; Devereux, M.; Enyedy, E.A.; Kia, A.F.A.; Karcz, D.; Kellett, A.; McClean, S.; Nagy, N.V.; Noble, A.; et al. Biological activity and coordination modes of copper(II) complexes of Schiff base-derived coumarin ligands. Dalton Trans. 2010, 39, 10854–10865. [Google Scholar] [CrossRef] [PubMed]
- Creaven, B.S.; Devereux, M.; Georgieva, I.; Karcz, D.; McCann, M.; Trendafilova, N.; Walsh, M. Molecular structure and spectroscopic studies on novel complexes of coumarin-3-carboxylic acid with Ni(II), Co(II), Zn(II) and Mn(II) ions based on density functional theory. Spectrochim. Acta A 2011, 84, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; Kia, A.F.-A.; Long, M.; Walsh, M.; Kavanagh, K.; McClean, S.; Creaven, B.S. Isolation and characterisation of silver(I) complexes of substituted coumarin-4-carboxylates which are effective against Pseudomonas aeruginosa biofilms. Polyhedron 2014, 67, 549–559. [Google Scholar] [CrossRef]
- Mujahid, M.; Kia, A.F.-A.; Duff, B.; Egan, D.A.; Devereux, M.; McClean, S.; Walsh, M.; Trendafilova, N.; Georgieva, I.; Creaven, B.S. Spectroscopic studies, DFT calculations, and cytotoxic activity of novel silver(I) complexes of hydroxy orthO− substituted-nitro-2H-chromen-2-one ligands and a phenanthroline adduct. J. Inorg. Biochem. 2015, 153, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Domotor, O.; Tuccinardi, T.; Karcz, D.; Walsh, M.; Creaven, B.S.; Enyedy, E.A. Interaction of anticancer reduced Schiff base coumarin derivatives with human serum albumin investigated by fluorescence quenching and molecular modeling. Bioorg. Chem. 2014, 52, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, S.; Bhattacharya, K.; Sullivan, M.; Walsh, M.; Creaven, B.S.; Laffir, F.; Duffy, B.; McHale, P. Non-cytotoxic antibacterial silver–coumarin complex doped sol–gel coatings. Colloids Surf. B Biointerfaces 2013, 102, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujahid, M.; Trendafilova, N.; Arfa-Kia, A.F.; Rosair, G.; Kavanagh, K.; Devereux, M.; Walsh, M.; McClean, S.; Creaven, B.S.; Georgieva, I. Novel silver(I) complexes of coumarin oxyacetate ligands and their phenanthroline adducts: Biological activity, structural and spectroscopic characterisation. J. Inorg. Biochem. 2016, 163, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Starzak, K.; Pietrzkowski, Z. Chlorination of Betacyanins in Several Hypochlorous Acid Systems. J. Agric. Food Chem. 2016, 64, 2865–2874. [Google Scholar] [CrossRef]
- Wybraniec, S.; Starzak, K.; Szneler, E.; Pietrzkowski, Z. Separation of chlorinated diastereomers of decarboxy-betacyanins in myeloperoxidase catalyzed chlorinated Beta vulgaris L. extract. J. Chromatogr. B 2016, 1036–1037, 20–32. [Google Scholar] [CrossRef]
- Wybraniec, S.; Starzak, K.; Skopinska, A.; Nemzer, B.; Pietrzkowski, Z.; Michalowski, T. Studies on nonenzymatic oxidation mechanisms in neobetanin, betanin, and decarboxylated betanins. J. Agric. Food Chem. 2013, 61, 6465–6476. [Google Scholar] [CrossRef]
- Wendel, M.; Nizinski, S.; Tuwalska, D.; Starzak, K.; Szot, D.; Prukala, D.; Sikorski, M.; Wybraniec, S.; Burdzinski, G. Time-resolved spectroscopy of the singlet excited state of betanin in aqueous and alcoholic solutions. Phys. Chem. Chem. Phys. 2015, 17, 18152–18158. [Google Scholar] [CrossRef] [PubMed]
- Wendel, M.; Szot, D.; Starzak, K.; Tuwalska, D.; Gapinski, J.; Naskrecki, R.; Prukala, D.; Sikorski, M.; Wybraniec, S.; Burdzinski, G. Photophysical properties of betaxanthins: Vulgaxanthin I in aqueous and alcoholic solutions. J. Lumin. 2015, 167, 289–295. [Google Scholar] [CrossRef]
- Wendel, M.; Szot, D.; Starzak, K.; Tuwalska, D.; Prukala, D.; Pedzinski, T.; Sikorski, M.; Wybraniec, S.; Burdzinski, G. Photophysical properties of indicaxanthin in aqueous and alcoholic solutions. Dyes Pigments 2015, 113, 634–639. [Google Scholar] [CrossRef]
- Wendel, M.; Nizinski, S.; Gierszewski, M.; Prukala, D.; Sikorski, M.; Starzak, K.; Wybraniec, S.; Burdzinski, G. Chemical quenching of singlet oxygen by betanin. Photochem. Photobiol. Sci. 2016, 15, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Karcz, D.; Matwijczuk, A.; Boroń, B.; Creaven, B.; Fiedor, L.; Niewiadomy, A.; Gagoś, M. Isolation and spectroscopic characterization of Zn(II), Cu(II), and Pd(II) complexes of 1,3,4-thiadiazole-derived ligand. J. Mol. Struct. 2017, 1128, 44–50. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Karcz, D.; Walkowiak, R.; Furso, J.; Gładyszewska, B.; Wybraniec, S.; Niewiadomy, A.; Karwasz, G.P.; Gagoś, M. Effect of Solvent Polarizability on the Keto/Enol Equilibrium of Selected Bioactive Molecules from the 1,3,4-Thiadiazole Group with a 2,4-Hydroxyphenyl Function. J. Phys. Chem. A 2017, 121, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Matwijczuk, A.; Kluczyk, D.; Górecki, A.; Niewiadomy, A.; Gagoś, M. Spectroscopic Studies of Fluorescence Effects in Bioactive 4-(5-Heptyl-1,3,4-Thiadiazol-2-yl)Benzene-1,3-Diol and 4-(5-Methyl-1,3,4-Thiadiazol-2-yl)Benzene-1,3-Diol Molecules Induced by pH Changes in Aqueous Solutions. J. Fluoresc. 2017, 27, 1201–1212. [Google Scholar] [CrossRef] [Green Version]
- Matwijczuk, A.; Karcz, D.; Pustuła, K.; Makowski, M.; Górecki, A.; Kluczyk, D.; Karpińska, M.M.; Niewiadomy, A.; Gagoś, M. Spectroscopic and theoretical studies of fluorescence effects in biO-active: 4-(5-(methyl-1,3,4-thiadiazol-2-yl))benzene-1,3-diol and 4-(5-(methylamino-1,3,4-thiadiazol-2-yl))benzene-1,3-diol compounds: Effect of molecular aggregation and amino group position. J. Luminesc. 2018, 201, 44–56. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Kluczyk, D.; Górecki, A.; Niewiadomy, A.; Gagoś, M. Solvent Effects on Molecular Aggregation in 4-(5-Heptyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol and 4-(5-Methyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol. J. Phys. Chem. B 2016, 120, 7958–7969. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Kamiński, D.; Górecki, A.; Ludwiczuk, A.; Niewiadomy, A.; Maćkowski, S.; Gagoś, M. Spectroscopic Studies of Dual Fluorescence in 2-((4-Fluorophenyl)amino)-5-(2,4-dihydroxybenzeno)-1,3,4-thiadiazole. J. Phys. Chem. A 2015, 119, 10791–10805. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Górecki, A.; Kamiński, D.; Myśliwa-Kurdziel, B.; Fiedor, L.; Niewiadomy, A.; Karwasz, G.P.; Gagoś, M. Influence of Solvent Polarizability on the KetO-Enol Equilibrium in 4-[5-(naphthalen-1-ylmethyl)-1,3,4-thiadiazol-2-yl]benzene-1,3-diol. J. Fluoresc. 2015, 25, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Hoser, A.A.; Kaminski, D.M.; Matwijczuk, A.; Niewiadomy, A.; Gagos, M.; Wozniak, K. On polymorphism of 2-(4-fluorophenylamino)-5-(2,4-dihydroxybenzeno)-1,3,4-thiadiazole (FABT) DMSO solvates. CrystEngComm 2013, 15, 1978–1988. [Google Scholar] [CrossRef]
- Gagoś, M.; Matwijczuk, A.; Kamiński, D.; Niewiadomy, A.; Kowalski, R.; Karwasz, G.P. Spectroscopic Studies of Intramolecular Proton Transfer in 2-(4-Fluorophenylamino)-5-(2,4-Dihydroxybenzeno)-1,3,4-Thiadiazole. J. Fluoresc. 2011, 21, 1–10. [Google Scholar] [CrossRef]
- Xiong, K.; Huo, F.; Zhang, Y.; Wen, Y.; Chao, J.; Yin, C. A novel recognition mechanism based on aldehyde group oxidized into carboxyl group by hypochlorite for the materials of fluorescent probes. Sens. Actuators B 2018, 255, 2378–2383. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Song, J.; Yang, Y. Development of an ICT-based ratiometric fluorescent hypochlorite probe suitable for living cell imaging. Chem. Commun. 2011, 47, 12691–12693. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.; Zhao, Y.; Fan, D.; Chen, L.; Qiu, Y.; Qian, L.; Zhang, K.; Yang, C. Crystal structure and photoluminescence of 7-(N,N-diethylamino)-coumarin-3-carboxylic acid. Spectrochim. Acta A 2008, 69, 1136–1139. [Google Scholar] [CrossRef]
- Chatterjee, A.; Seth, D. Photophysical Properties of 7-(diethylamino)Coumarin-3-carboxylic Acid in the Nanocage of Cyclodextrins and in Different Solvents and Solvent Mixtures. Photochem. Photobiol. 2013, 89, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Sanap, K.K.; Samant, S.D. Synthesis of coumarin based fluorescent compounds. Tetrahedron Lett. 2012, 53, 5407–5410. [Google Scholar] [CrossRef]
- Yap, Y.W.; Whiteman, M.; Cheung, N.S. Chlorinative stress: An under appreciated mediator of neurodegeneration? Cell Signal. 2007, 19, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukocyte Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.J.; Albrett, A.M.; Chapman, A.L.; Dickerhof, N.; Forbes, L.V.; Khalilova, I.; Turner, R. Measuring chlorine bleach in biology and medicine. Biochim. Biophys. Acta 2014, 1840, 781–793. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food. Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Brewer, W.D.; Haken, H.; Wolf, H.C. Molecular Physics and Elements of Quantum Chemistry: Introduction to Experiments and Theory; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Liu, S.-R.; Wu, S.-P. Hypochlorous Acid Turn-on Fluorescent Probe Based on Oxidation of Diphenyl Selenide. Org. Lett. 2013, 15, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huo, F.; Zhang, J.; Xie, Z.; Chao, J.; Yin, C.; Tong, H.; Liu, D.; Jin, S.; Cheng, F.; et al. A novel coumarin-based fluorescent probe for selective detection of bissulfite anions in water and sugar samples. Sens. Actuators B 2012, 166–167, 665–670. [Google Scholar] [CrossRef]
- Teizo, S.; Koichi, T. Solvent-Free Coumarin Synthesis. Chem. Lett. 2001, 30, 110–111. [Google Scholar] [CrossRef] [Green Version]
- Maggi, F.; Barboni, L.; Caprioli, G.; Papa, F.; Ricciutelli, M.; Sagratini, G.; Vittori, S. HPLC quantification of coumarin in bastard balm (Melittis melissophyllum L., Lamiaceae). Fitoterapia 2011, 82, 1215–1221. [Google Scholar] [CrossRef]
- Krieger, S.; Hayen, H.; Schmitz, O.J. Quantification of coumarin in cinnamon and woodruff beverages using DIP-APCI-MS and LC-MS. Anal. Bioanal. Chem. 2013, 405, 8337–8345. [Google Scholar] [CrossRef]
- Ren, Z.; Nie, B.; Liu, T.; Yuan, F.; Feng, F.; Zhang, Y.; Zhou, W.; Xu, X.; Yao, M.; Zhang, F. Simultaneous Determination of Coumarin and Its Derivatives in Tobacco Products by Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2016, 21, 1511. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Z.; Zhou, A.; Meng, M.; Li, Q. Simultaneous analysis of coumarin derivatives in extracts of Radix Angelicae pubescentis (Duhuo) by HPLC-DAD-ESI-MSn technique. Anal. Methods 2014, 6, 7996–8002. [Google Scholar] [CrossRef]







| Compound No. | Compound Name | tret (min) | λmax (nm) | m/z [M+H]+ | Composition (%) |
|---|---|---|---|---|---|
| 1 | 7-diethylamino-3-formylcoumarin | 7.9 | 443 | 246.05 | 29.1 |
| 1a′ | Monochloro-7-diethylamino-3-formylcoumarin * | 9.7 | 433 | 279.95 | 25.8 |
| 1a′′ | monochloro-7-diethylamino-3-formylcoumarin * | 11.1 | 440 | 279.05 | 2.5 |
| 1b | dichloro-7-diethylaminocoumarin * | 12.1 | 366 | 285.95 | 4.2 |
| 2 | 7-diethylaminocoumarin-3-carboxylic acid | 8.1 | 432 | 262.00 | 15.6 |
| 2a′ | monochloro-7-diethylaminocoumarin-3-carboxylic acid * | 9.9 | 411 | 295.95 | 33.5 |
| 2a′′ | monochloro-7-diethylaminocoumarin-3-carboxylic acid * | 10.9 | 396 | 295.95 | 0.5 |
| 1b | dichloro-7-diethylaminocoumarin * | 12.1 | 366 | 285.90 | 5.9 |
| 3a′ | monochloro-7-diethylamino-4-methylcoumarin * | 10.2 | 350 | 266.00 | 44.3 |
| 3 | 7-diethylamino-4-methylcoumarin | 10.5 | 375 | 232.05 | 27.2 |
| 3a′′ | monochloro-7-diethylamino-4-methylcoumarin * | 12.9 | 389 | 266.00 | 19.1 |
| 3b | dichloro-7-diethylamino-4-methylcoumarin * | 13.3 | 360 | 299.95 | 6.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starzak, K.; Matwijczuk, A.; Creaven, B.; Matwijczuk, A.; Wybraniec, S.; Karcz, D. Fluorescence Quenching-Based Mechanism for Determination of Hypochlorite by Coumarin-Derived Sensors. Int. J. Mol. Sci. 2019, 20, 281. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20020281
Starzak K, Matwijczuk A, Creaven B, Matwijczuk A, Wybraniec S, Karcz D. Fluorescence Quenching-Based Mechanism for Determination of Hypochlorite by Coumarin-Derived Sensors. International Journal of Molecular Sciences. 2019; 20(2):281. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20020281
Chicago/Turabian StyleStarzak, Karolina, Arkadiusz Matwijczuk, Bernadette Creaven, Alicja Matwijczuk, Sławomir Wybraniec, and Dariusz Karcz. 2019. "Fluorescence Quenching-Based Mechanism for Determination of Hypochlorite by Coumarin-Derived Sensors" International Journal of Molecular Sciences 20, no. 2: 281. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20020281