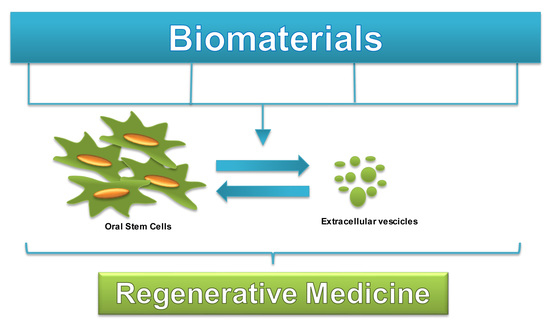
Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair
Abstract
:1. Introduction
2. Morphological Features and Phenotypic Characterization of Human Oral Stem Cells
3. Biogenesis and Characterization of EVs
4. Clinical Potential and Current Progress of Biomaterials and Human Oral Stem Cells in Bone Tissue Regeneration
5. Conclusions
Funding
Conflicts of Interest
References
- Florencio-Silva, R.; Sasso, G.R.D.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Coleman, R. Aging and bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Calejo, I.; Costa-Almeida, R.; Gomes, M.E. Cellular Complexity at the Interface: Challenges in Enthesis Tissue Engineering. Cell Biol. Transl. Med. 2019, 1144, 71–90. [Google Scholar]
- Paduano, F.; Marrelli, M.; Amantea, M.; Rengo, C.; Rengo, S.; Goldberg, M.; Spagnuolo, G.; Tatullo, M. Adipose Tissue as a Strategic Source of Mesenchymal Stem Cells in Bone Regeneration: A Topical Review on the Most Promising Craniomaxillofacial Applications. Int. J. Mol. Sci. 2017, 18, 2140. [Google Scholar] [CrossRef] [PubMed]
- Nocca, G.; Calla, C.; Martorana, G.E.; Cicillini, L.; Rengo, S.; Lupi, A.; Cordaro, M.; Gozzo, M.L.; Spagnuolo, G. Effects of Dental Methacrylates on Oxygen Consumption and Redox Status of Human Pulp Cells. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent. J. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Ibarretxe, G.; Crende, O.; Aurrekoetxea, M.; Garcia-Murga, V.; Etxaniz, J.; Unda, F. Neural crest stem cells from dental tissues: A new hope for dental and neural regeneration. Stem Cells Int. 2012, 2012, 103503. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manrique, P.; Matos, M.; Gutierrez, G.; Pazos, C.; Blanco-Lopez, M.C. Therapeutic biomaterials based on extracellular vesicles: Classification of bio-engineering and mimetic preparation routes. J. Extracell Vesicles 2018, 7, page. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, page. [Google Scholar] [CrossRef]
- Trubiani, O.; Toniato, E.; Di Iorio, D.; Diomede, F.; Merciaro, I.; D’Arcangelo, C.; Caputi, S. Morphological Analysis and Interleukin Release in Human Gingival Fibroblasts Seeded on Different Denture Base Acrylic Resins. Int. J. Immunopath. Ph. 2012, 25, 637–643. [Google Scholar] [CrossRef]
- Diomede, F.; Zini, N.; Pizzicannella, J.; Merciaro, I.; Pizzicannella, G.; D’Orazio, M.; Piattelli, A.; Trubiani, O. 5-Aza Exposure Improves Reprogramming Process Through Embryoid Body Formation in Human Gingival Stem Cells. Front Genet 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trubiani, O.; Ballerini, P.; Murmura, G.; Pizzicannella, J.; Giuliani, P.; Buccella, S.; Caputi, S. Toll-Like Receptor 4 Expression, Interleukin-6,-8 and Ccl-20 Release, and Nf-Kb Translocation in Human Periodontal Ligament Mesenchymal Stem Cells Stimulated with Lps-P-Gingivalis. Eur. J. Inflamm. 2012, 10, 81–89. [Google Scholar] [CrossRef]
- Park, Y.J.; Cha, S.H.; Park, Y.S. Regenerative Applications Using Tooth Derived Stem Cells in Other Than Tooth Regeneration: A Literature Review. Stem Cells Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Gronthos, S.; Shi, S.; Bartold, P.M. Stem cells in the periodontal ligament. Oral Dis. 2006, 12, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Avinash, K.; Malaippan, S.; Dooraiswamy, J.N. Methods of Isolation and Characterization of Stem Cells from Different Regions of Oral Cavity Using Markers: A Systematic Review. Int. J. Stem Cells 2017, 10, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Marrelli, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Odontogenic Differentiation of Human Dental Pulp Stem Cells on Hydrogel Scaffolds Derived from Decellularized Bone Extracellular Matrix and Collagen Type I. PloS ONE 2016, 11, e0148225. [Google Scholar] [CrossRef] [PubMed]
- Chase, L.G.; Lakshmipathy, U.; Solchaga, L.A.; Rao, M.S.; Vemuri, M.C. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res. Ther. 2010, 1, page. [Google Scholar] [CrossRef] [PubMed]
- Banfi, A.; Muraglia, A.; Dozin, B.; Mastrogiacomo, M.; Cancedda, R.; Quarto, R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 2000, 28, 707–715. [Google Scholar] [CrossRef]
- Salvade, A.; Belotti, D.; Donzelli, E.; D’Amico, G.; Gaipa, G.; Renoldi, G.; Carini, F.; Baldoni, M.; Pogliani, E.M.; Tredici, G.; et al. GMP-grade preparation of biomimetic scaffolds with osteo-differentiated autologous mesenchymal stromal cells for the treatment of alveolar bone resorption in periodontal disease. Cytotherapy 2007, 9, 427–438. [Google Scholar] [CrossRef]
- Yu, J.; He, H.; Tang, C.; Zhang, G.; Li, Y.; Wang, R.; Shi, J.; Jin, Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010, 11, 32. [Google Scholar] [CrossRef]
- Diomede, F.; Rajan, T.S.; Gatta, V.; D’Aurora, M.; Merciaro, I.; Marchisio, M.; Muttini, A.; Caputi, S.; Bramanti, P.; Mazzon, E.; et al. Stemness Maintenance Properties in Human Oral Stem Cells after Long-Term Passage. Stem Cells Int. 2017, 2017, 5651287. [Google Scholar] [CrossRef] [PubMed]
- d’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ 2007, 14, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, M.; Indumathi, S.; Lissa, R.P.; Harikrishnan, R.; Rajkumar, J.S.; Sudarsanam, D. A comprehensive study on optimization of proliferation and differentiation potency of bone marrow derived mesenchymal stem cells under prolonged culture condition. Cytotechnology 2013, 65, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Menicanin, D.; Mrozik, K.M.; Wada, N.; Marino, V.; Shi, S.T.; Bartold, P.M.; Gronthos, S. Periodontal-Ligament-Derived Stem Cells Exhibit the Capacity for Long-Term Survival, Self-Renewal, and Regeneration of Multiple Tissue Types in Vivo. Stem Cells Dev. 2014, 23, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Menicanin, D.; Bartold, P.M.; Zannettino, A.C.W.; Gronthos, S. Identification of a Common Gene Expression Signature Associated with Immature Clonal Mesenchymal Cell Populations Derived from Bone Marrow and Dental Tissues. Stem Cells Dev. 2010, 19, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Pan, K.; Sun, Q.; Zhang, J.; Ge, S.; Li, S.; Zhao, Y.; Yang, P. Multilineage differentiation of dental follicle cells and the roles of Runx2 over-expression in enhancing osteoblast/cementoblast-related gene expression in dental follicle cells. Cell Proliferat 2010, 43, 219–228. [Google Scholar] [CrossRef]
- Mammana, S.; Gugliandolo, A.; Cavalli, E.; Diomede, F.; Iori, R.; Zappacosta, R.; Bramanti, P.; Conti, P.; Fontana, A.; Pizzicannella, J.; et al. Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J. Tissue Eng. Regen. Med. 2019. [Google Scholar] [CrossRef]
- Maeda, T.; Ochi, K.; Nakakura-Ohshima, K.; Youn, S.H.; Wakisaka, S. The Ruffini ending as the primary mechanoreceptor in the periodontal ligament: Its morphology, cytochemical features, regeneration, and development. Crit. Rev. Oral Biol. M. 1999, 10, 307–327. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Diomede, F.; Merciaro, I.; Caputi, S.; Tartaro, A.; Guarnieri, S.; Trubiani, O. Endothelial committed oral stem cells as modelling in the relationship between periodontal and cardiovascular disease. J. Cell. Physiol. 2018, 233, 6734–6747. [Google Scholar] [CrossRef]
- Lee, J.S.; An, S.Y.; Kwon, I.K.; Heo, J.S. Transdifferentiation of human periodontal ligament stem cells into pancreatic cell lineage. Cell Biochem Funct. 2014, 32, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.; Yung, J.S.Y.; Choy, K.W.; Cao, D.; Leung, C.K.S.; Cheung, H.S.; Pang, C.P. Transdifferentiation of periodontal ligament-derived stem cells into retinal ganglion-like cells and its microRNA signature. Sci. Rep. 2015, 5, 16429. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Merciaro, I.; Martinotti, S.; Cavalcanti, M.F.X.B.; Caputi, S.; Mazzon, E.; Trubiani, O. miR-2861 IS INVOLVED IN OSTEOGENIC COMMITMENT OF HUMAN PERIODONTAL LIGAMENT STEM CELLS GROWN ONTO 3D SCAFFOLD. J. Biol. Regul. Homeost. Agents 2016, 30, 1009–1018. [Google Scholar] [PubMed]
- Umezaki, Y.; Hashimoto, Y.; Nishishita, N.; Kawamata, S.; Baba, S. Human Gingival Integration-Free iPSCs; a Source for MSC-Like Cell. Int. J. Mol. Sci. 2015, 16, 13633–13648. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Wang, B.; Lin, N.H.; Laslett, A.L.; Gronthos, S.; Bartold, P.M. Induced pluripotent stem cell lines derived from human gingival fibroblasts and periodontal ligament fibroblasts. J. Periodontal. Res. 2011, 46, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Hou, Y.; Song, P.; Zhou, M.; Nie, M.; Liu, X. Modulation of proliferation and differentiation of gingivaderived mesenchymal stem cells by concentrated growth factors: Potential implications in tissue engineering for dental regeneration and repair. Int. J. Mol. Med. 2019, 44, 37–46. [Google Scholar] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Pizzicannella, J.; Gugliandolo, A.; Orsini, T.; Fontana, A.; Ventrella, A.; Mazzon, E.; Bramanti, P.; Diomede, F.; Trubiani, O. Engineered Extracellular Vesicles From Human Periodontal-Ligament Stem Cells Increase VEGF/VEGFR2 Expression During Bone Regeneration. Front. Physiol. 2019, 10, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beez, C.M.; Haag, M.; Klein, O.; Van Linthout, S.; Sittinger, M.; Seifert, M. Extracellular vesicles from regenerative human cardiac cells act as potent immune modulators by priming monocytes. J. Nanobiotechnol. 2019, 17, 72. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, Y.; Zhang, Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J. Dent. Res. 2018, 97, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yeo, J.C.; Lim, C.T. Advances in Technologies for Purification and Enrichment of Extracellular Vesicles. SLAS Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Odorizzi, G. The multiple personalities of Alix. J. Cell Sci. 2006, 119, 3025–3032. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Rasool, H.I.; Palanisamy, V.; Mathisen, C.; Schmidt, M.; Wong, D.T.; Gimzewski, J.K. Structural-Mechanical Characterization of Nanoparticle Exosomes in Human Saliva, Using Correlative AFM, FESEM, and Force Spectroscopy. Acs Nano 2010, 4, 1921–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Xu, N.; Zhang, Z.L.; Zhang, T.C. Cell derived extracellular vesicles: From isolation to functionalization and biomedical applications. Biomater. Sci. 2019, 7, 3552–3565. [Google Scholar] [CrossRef]
- Diomede, F.; D’Aurora, M.; Gugliandolo, A.; Merciaro, I.; Ettorre, V.; Bramanti, A.; Piattelli, A.; Gatta, V.; Mazzon, E.; Fontana, A.; et al. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int. J. Nanomed. 2018, 13, 3805–3825. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Kaplan, D.L. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev. Biol. 2009, 20, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Scioli, M.G.; Bielli, A.; Gentile, P.; Cervelli, V.; Orlandi, A. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 2398–2410. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.S.; Rayan, F.; Dhinsa, B.S.; Marsh, D. An Osteoconductive, Osteoinductive, and Osteogenic Tissue-Engineered Product for Trauma and Orthopaedic Surgery: How Far Are We? Stem Cells Int. 2012, Unsp 236231. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Lucarini, L.; Spallone, D.; Palla, L.; Colicchia, G.M.; Gentile, P.; De Angelis, B. Use of platelet-rich plasma and hyaluronic acid in the loss of substance with bone exposure. Adv. Skin Wound Care 2011, 24, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Schmelzeisen, R.; Schimming, R.; Sittinger, M. Making bone: Implant insertion into tissue-engineered bone for maxillary sinus floor augmentation - a preliminary report. J. Cranio Maxill Surg. 2003, 31, 34–39. [Google Scholar] [CrossRef]
- Sun, J.; Vijayavenkataraman, S.; Liu, H. An Overview of Scaffold Design and Fabrication Technology for Engineered Knee Meniscus. Materials 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Shuo, Z.; Fuh, J.Y.H.; Lu, W.F. Design of Three-Dimensional Scaffolds with Tunable Matrix Stiffness for Directing Stem Cell Lineage Specification: An In Silico Study. Bioengineering 2017, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef]
- Maeda, M.; Tani, S.; Sano, A.; Fujioka, K. Microstructure and release characteristics of the minipellet, a collagen-based drug delivery system for controlled release of protein drugs. J. Control. 1999, 62, 313–324. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Rosenthal, F.M.; Kohler, G. Collagen as matrix for neo-organ formation by gene-transfected fibroblasts. Anticancer Res. 1997, 17, 1179–1186. [Google Scholar] [PubMed]
- Doillon, C.J.; Silver, F.H. Collagen-Based Wound Dressing - Effects of Hyaluronic-Acid and Fibronectin on Wound-Healing. Biomaterials 1986, 7, 3–8. [Google Scholar] [CrossRef]
- Park, J.Y.; Shim, J.H.; Choi, S.A.; Jang, J.; Kim, M.; Lee, S.H.; Cho, D.W. 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J. Mater Chem. B 2015, 3, 5415–5425. [Google Scholar] [CrossRef] [Green Version]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Diomede, F.; Cardelli, P.; Bramanti, A.; Scionti, D.; Bramanti, P.; Trubiani, O.; Mazzon, E. Transcriptomic analysis of gingival mesenchymal stem cells cultured on 3D bioprinted scaffold: A promising strategy for neuroregeneration. J. Biomed. Mater. Res. 2018, 106, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Shum, A.W.T.; Mak, A.F.T. Morphological and biomechanical characterization of poly(glycolic acid) scaffolds after in vitro degradation. Polym Degrad Stabil 2003, 81, 141–149. [Google Scholar] [CrossRef]
- Lopes, M.S.; Jardini, A.L.; Maciel, R. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Adachi, T.; Osako, Y.; Tanaka, M.; Hojo, M.; Hollister, S.J. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials 2006, 27, 3964–3972. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Scionti, D.; Merciaro, I.; Cavalcanti, M.F.; Mazzon, E.; Trubiani, O. Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration. Int. J. Mol. Sci. 2018, 19, 329. [Google Scholar] [CrossRef]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef]
- Taguchi, Y.; Amizuka, N.; Nakadate, M.; Ohnishi, H.; Fujii, N.; Oda, K.; Nomura, S.; Maeda, T. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials 2005, 26, 6158–6166. [Google Scholar] [CrossRef] [PubMed]
- Postlethwaite, A.E.; Seyer, J.M.; Kang, A.H. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc. Natl. Acad. Sci. USA 1978, 75, 871–875. [Google Scholar] [CrossRef]
- Guirado, J.L.C.; Fernandez, M.P.R.; Negri, B.; Ruiz, R.A.D.; de-Val, J.E.M.S.; Gomez-Moreno, G. Experimental Model of Bone Response to Collagenized Xenografts of Porcine Origin (OsteoBiol (R) mp3): A Radiological and Histomorphometric Study. Clin Implant Dent. R 2013, 15, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Malmstrom, H.; Ren, Y.F. Porous Collagen-Hydroxyapatite Scaffolds With Mesenchymal Stem Cells for Bone Regeneration. J. Oral Implantol. 2015, 41, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, B.X.; Cui, Z.; Cui, S.J.; Zhang, T.; Wang, X.D.; Liu, D.W.; Yang, R.L.; Jiang, N.; Zhou, Y.H.; et al. Bone regeneration in minipigs by intrafibrillarly-mineralized collagen loaded with autologous periodontal ligament stem cells. Sci. Rep.-Uk 2017, 7, 10519. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Luan, J.; Li, H.; Zhou, X.; Han, J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016, 590, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liu, X.L.; Li, H.Y.; Chen, C.Y.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.Z.; Xie, Z.P.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Zuber, G.; Dauty, E.; Nothisen, M.; Belguise, P.; Behr, J.P. Towards synthetic viruses. Adv. Drug Deliver. Rev. 2001, 52, 245–253. [Google Scholar] [CrossRef]
- Beyer, S.; Fleming, J.; Meng, W.; Singh, R.; Haque, S.J.; Chakravarti, A. The Role of miRNAs in Angiogenesis, Invasion and Metabolism and Their Therapeutic Implications in Gliomas. Cancers 2017, 9, 7. [Google Scholar]
- Pizzicannella, J.; Cavalcanti, M.; Trubiani, O.; Diomede, F. MicroRNA 210 Mediates VEGF Upregulation in Human Periodontal Ligament Stem Cells Cultured on 3DHydroxyapatite Ceramic Scaffold. Int. J. Mol. Sci. 2018, 19, 3916. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20204987
Trubiani O, Marconi GD, Pierdomenico SD, Piattelli A, Diomede F, Pizzicannella J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. International Journal of Molecular Sciences. 2019; 20(20):4987. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20204987
Chicago/Turabian StyleTrubiani, Oriana, Guya D. Marconi, Sante D. Pierdomenico, Adriano Piattelli, Francesca Diomede, and Jacopo Pizzicannella. 2019. "Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair" International Journal of Molecular Sciences 20, no. 20: 4987. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20204987