Improved Electrophoretic Separation to Assist the Monitoring of Bcl-xL Post-Translational Modifications
Abstract
:1. Introduction
2. Results
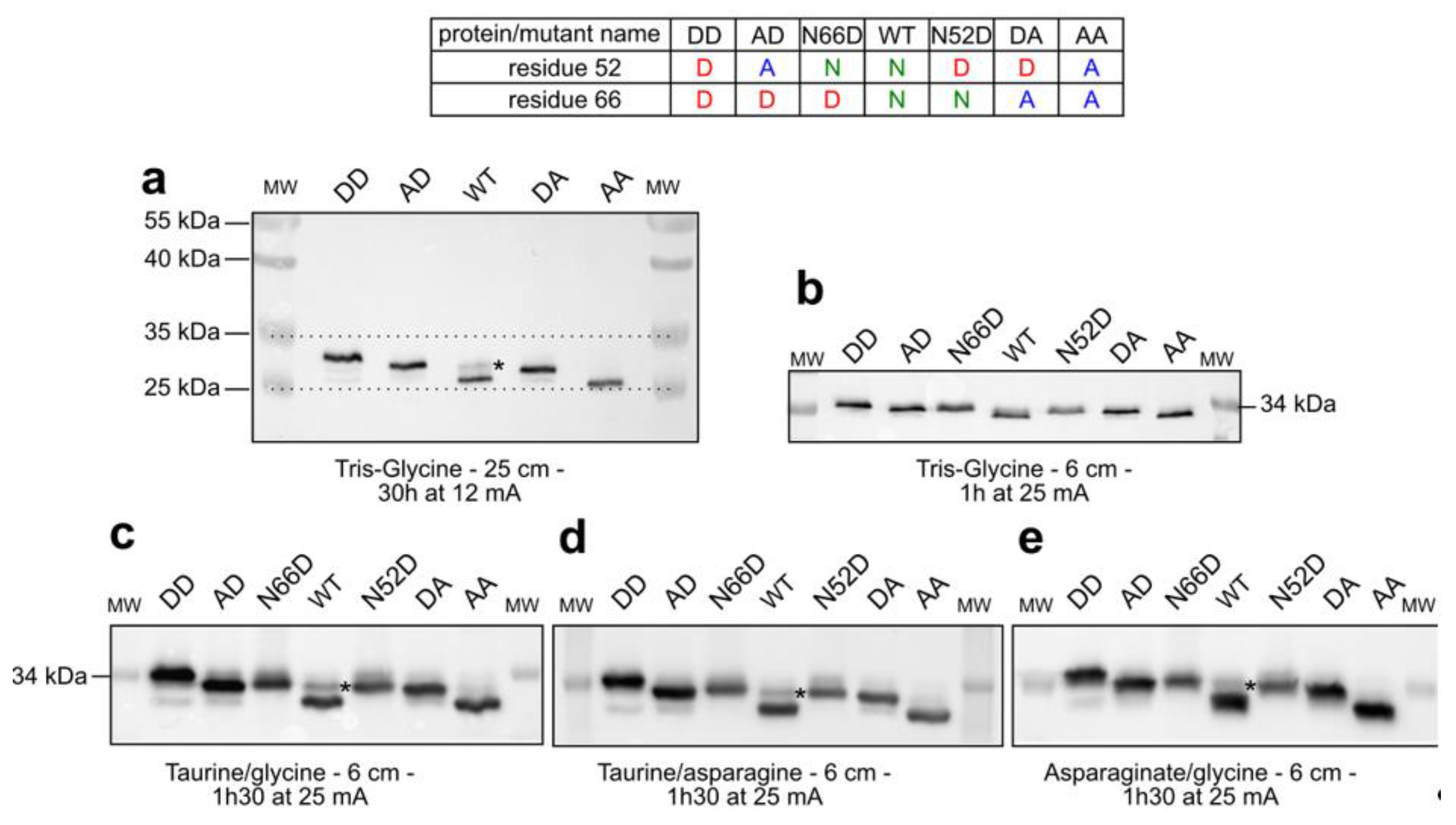
2.1. Separation of Bcl-xL Deamido-Mimetic Mutants
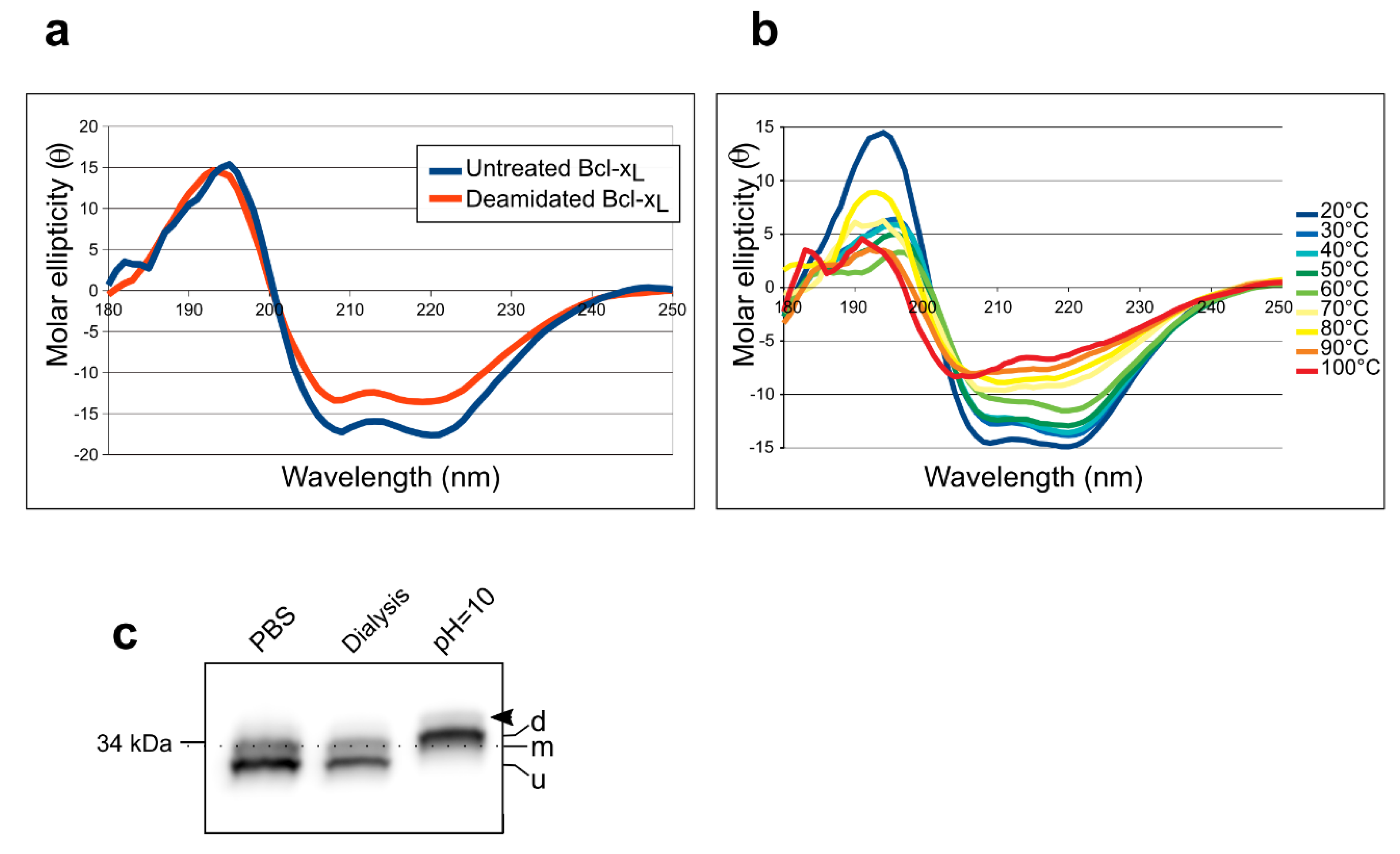
2.2. Separation of Chemically-Induced Deamidated Bcl-xL
2.3. Detection of Accumulated PTMs.
3. Discussion
3.1. Assisting the Detection of Deamidation
3.2. Evidence for Accumulation of PTMs by Bcl-xL
3.3. Deamidation of Bcl-xL: Open Questions and Applications
4. Materials and Methods
4.1. Recombinant Proteins
4.2. Electrophoresis
4.3. Circular Dichroism
4.4. Alkaline Treatment
4.5. Cell Lines and Culture
4.6. Total Proteins Extraction
4.7. Lambda Phosphatase Treatment
4.8. Western Blots
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arbel, N.; Ben-Hail, D.; Shoshan-Barmatz, V. Mediation of the Antiapoptotic Activity of Bcl-xL Protein upon Interaction with VDAC1 Protein. J. Biol. Chem. 2012, 287, 23152–23161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petros, A.M.; Gunasekera, A.; Xu, N.; Olejniczak, E.T.; Fesik, S.W. Defining the p53 DNA-binding domain/Bcl-xL-binding interface using NMR. FEBS Lett. 2004, 559, 171–174. [Google Scholar] [CrossRef]
- Trécesson, S.D.; Souazé, F.; Basseville, A.; Bernard, A.-C.; Pécot, J.; Lopez, J.; Bessou, M.; Sarosiek, K.A.; Letain, A.; Barillé-Nion, B.-N.; et al. BCL-XL directly modulates RAS signalling to favour cancer cell stemness. Nat. Commun. 2017, 8, 1123. [Google Scholar] [CrossRef] [PubMed]
- Follis, A.V.; Llambi, F.; Kalkavan, H.; Yao, Y.; Phillips, A.H.; Park, C.-G.; Marassi, F.M.; Green, D.R.; Kriwacki, R.W. Regulation of apoptosis by an intrinsically disordered region of Bcl-xL. Nat. Chem. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hagn, F.; Klein, C.; Demmer, O.; Marchenko, N.; Vaseva, A.; Moll, U.M.; Kessler, H. BclxL Changes Conformation upon Binding to Wild-type but Not Mutant p53 DNA Binding Domain. J. Biol. Chem. 2010, 285, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Beaumatin, F.; el Dhaybi, M.; Bobo, C.; Verdier, M.; Priault, M. Bcl-xL deamidation and cancer: Charting the fame trajectories of legitimate child and hidden siblings. Biochim. Biophys. Acta 2017. [Google Scholar] [CrossRef] [PubMed]
- Beaumatin, F.; Dhaybi, M.E.; Lasserre, J.-P.; Salin, B.; Moyer, M.P.; Verdier, M.; Manon, S.; Priault, M. N52 monodeamidated Bcl-xL shows impaired oncogenic properties in vivo and in vitro. Oncotarget 2016, 7, 17129–17143. [Google Scholar] [CrossRef]
- Upreti, M.; Galitovskaya, E.N.; Chu, R.; Tackett, A.J.; Terrano, D.T.; Granell, S.; Chambers, T.C. Identification of the Major Phosphorylation Site in Bcl-xL Induced by Microtubule Inhibitors and Analysis of Its Functional Significance. J. Biol. Chem. 2008, 283, 35517–35525. [Google Scholar] [CrossRef] [Green Version]
- Ehlermann, P.; Redweik, U.; Blau, N.; Heizmann, C.W.; Katus, H.A.; Remppis, A. Separation of low molecular weight proteins with SDS-PAGE using taurine as a new trailing ion. Gen. Physiol. Biophys. 2001, 20, 203–207. [Google Scholar] [PubMed]
- Meinwald, Y.C.; Stimson, E.R.; Scheraga, H.A. Deamidation of the asparaginyl-glycyl sequence. Int. J. Pept. Protein Res. 1986, 28, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Borchardt, R.T. Chemical pathways of peptide degradation. II. Kinetics of deamidation of an asparaginyl residue in a model hexapeptide. Pharm. Res. 1990, 7, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Poruchynsky, M.S.; Wang, E.E.; Rudin, C.M.; Blagosklonny, M.V.; Fojo, T. Bcl-xL Is Phosphorylated in Malignant Cells following Microtubule Disruption. Cancer Res. 1998, 58, 3331–3338. [Google Scholar] [PubMed]
- Lorenzo, J.R.; Alonso, L.G.; Sánchez, I.E. Prediction of Spontaneous Protein Deamidation from Sequence-Derived Secondary Structure and Intrinsic Disorder. PLoS ONE 2015, 10, e0145186. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Nisan, D.; Fujimoto, L.M.; Antignani, A.; Barnes, A.; Tjandra, N.; Youle, R.J.; Marassi, F.M. Characterization of the membrane-inserted C-terminus of cytoprotective BCL-XL. Protein Expr. Purif. 2016, 122, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, L.; Wallace, B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, J.G.; Miles, A.J.; Wien, F.; Wallace, B.A. A reference database for circular dichroism spectroscopy covering fold and secondary structure space. Bioinformatics 2006, 22, 1955–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priault, M.; Hue, E.; Marhuenda, F.; Pilet, P.; Oliver, L.; Vallette, F.M. Differential Dependence on Beclin 1 for the Regulation of Pro-Survival Autophagy by Bcl-2 and Bcl-xL in HCT116 Colorectal Cancer Cells. PLoS ONE 2010, 5, e8755. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobo, C.; Céré, C.; Dufossée, M.; Dautant, A.; Moreau, V.; Manon, S.; Beaumatin, F.; Priault, M. Improved Electrophoretic Separation to Assist the Monitoring of Bcl-xL Post-Translational Modifications. Int. J. Mol. Sci. 2019, 20, 5571. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225571
Bobo C, Céré C, Dufossée M, Dautant A, Moreau V, Manon S, Beaumatin F, Priault M. Improved Electrophoretic Separation to Assist the Monitoring of Bcl-xL Post-Translational Modifications. International Journal of Molecular Sciences. 2019; 20(22):5571. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225571
Chicago/Turabian StyleBobo, Claude, Claire Céré, Mélody Dufossée, Alain Dautant, Violaine Moreau, Stéphen Manon, Florian Beaumatin, and Muriel Priault. 2019. "Improved Electrophoretic Separation to Assist the Monitoring of Bcl-xL Post-Translational Modifications" International Journal of Molecular Sciences 20, no. 22: 5571. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225571




