Titania Nanofiber Scaffolds with Enhanced Biointegration Activity—Preliminary In Vitro Studies
Abstract
:1. Introduction
2. Results
2.1. Morphology and Structure Characterization of Titania Nanocoatings
2.2. The Wettability and Surface Free Energy
2.3. The Roughness of the of Titania Nanocoatings
2.4. The Nanomechanical Properties of the Titania Nanocoatings
2.5. Cell Proliferation Determined by the MTT Assay
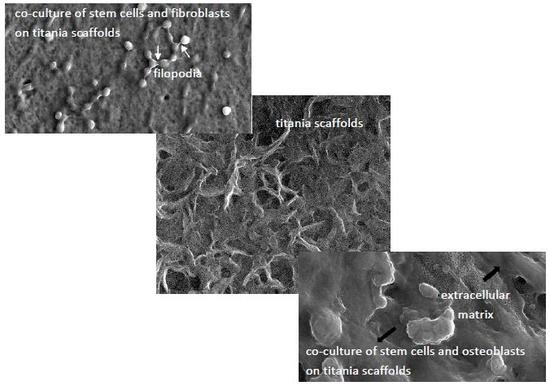
2.6. Cell Morphology Analyzed by Scanning Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Synthesis of Titania Nanocoatings
4.1.1. Titania Nanofibers (TNF4-10)
4.1.2. Titania Nanofibers (TNF72)
4.2. Characterization of Titania Nanocoatings
4.2.1. Structure and Morphology Characterization
4.2.2. The Wettability and Surface Free Energy
4.2.3. Mechanical Properties
4.3. Biological Studies
4.3.1. Cell Culture
4.3.2. Cell Proliferation Assays
4.3.3. Cell Morphology Observed by Scanning Electron Microscopy
4.3.4. Statistical Analysis in the MTT Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cowden, K.; Dias-Netipanyj, M.F.; Popat, K.C. Effects of titania nanotube surfaces on osteogenic differentiation of human adipose-derived stem cells. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Babaie, E.; Bhaduri, S.B. Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2018, 4, 1–39. [Google Scholar] [CrossRef]
- Singla, R.; Abidi, S.M.S.; Dar, A.I.; Acharya, A. Nanomaterials as potential and versatile platform for next generation tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Orapiriyakul, W.; Young, P.S.; Damiati, L.; Tsimbouri, P.M. Antibacterial surface modification of titanium implants in orthopaedics. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Jędrzejewski, T.; Wypij, M.; Golińska, P. “To Be Microbiocidal and Not to Be Cytotoxic at the Same Time…”—Silver Nanoparticles and Their Main Role on the Surface of Titanium Alloy Implants. J. Clin. Med. 2019, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Chu, P.K.; Ding, C.X. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Ingrassia, D.; Sladkova, M.; Palmer, M.; Xia, W.; Engqvist, H.; de Peppo, G.M. Stem cell-mediated functionalization of titanium implants. J. Mater. Sci. Mater. Med. 2017, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- Malec, K.; Góralska, J.; Hubalewska-Mazgaj, M.; Głowacz, P.; Jarosz, M.; Brzewski, P.; Sulka, G.D.; Jaskuła, M.; Wybrańska, J. Effects of nanoporous anodic titanium oxide on human adipose derived stem cells. Int. J. Nanomed. 2016, 11, 5349–5360. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Webster, T. Nanomedicine for implants: A review of studies and necessary experimental tools. Biomaterials 2007, 28, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Luzi, E.; Fabbri, S.; Ciuffi, S.; Sorace, S.; Tognarini, I.; Galli, G.; Zonefrati, R.; Sbaiz, F.; Brandi, M.L. Osteogenic differentiation of adipose tissue-derived mesenchymal stem cells on nanostructured Ti6Al4V and Ti13Nb13Zr. Clin. Cases Miner. Bone Metab. 2015, 12, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Dias-Netipanyj, M.F.; Cowden, K.; Sopchenski, L.; Cogo, S.C.; Elifio-Esposito, S.; Popat, K.C.; Soares, P. Effect of crystalline phases of titania nanotube arrays on adipose derived stem cell adhesion and proliferation. Mater. Sci. Eng. C 2019, 103, 109850. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Sbricoli, L.; Guazzo, R.; Tocco, I.; Roman, M.; Vindigni, V.; Stellini, E.; Gardin, C.; Ferroni, L.; Sivolella, S.; et al. Nanostructured Surfaces of Dental Implants. Int. J. Mol. Sci. 2013, 14, 1918–1931. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N.; Jin, M.; Zheng, Y.; Guan, Y.; Lu, X.; Luo, J.L. Nanotubular surface modification of metallic implants via electrochemical anodization technique. Int. J. Nanomed. 2014, 9, 4421–4435. [Google Scholar] [CrossRef] [PubMed]
- Tuukkanen, J.; Nakamura, M. Hydroxyapatite as a Nanomaterial for Advanced Tissue Engineering and Drug Therapy. Curr. Pharm. Des. 2017, 23, 3786–3793. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lin, Y.; Liu, Y.; Zhou, Y.; Liu, C.; Dong, L.; Cheng, K.; Weng, W.; Wang, H. Enhanced Osteointegration of Hierarchical Structured 3D-Printed Titanium Implants. ACS Appl. Bio Mater. 2018, 1, 90–99. [Google Scholar]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sadowska, B.; Więckowska-Szakiel, M.; Holopainen, J.; Ritala, M.; Leskelä, M.; Bartmański, M.; Szkodo, M.; et al. Titania Nanotubes/Hydroxyapatite Nanocomposites Produced with the Use of the Atomic Layer Deposition Technique: Estimation of Bioactivity and Nanomechanical Properties. Nanomaterials 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Bartmański, M. The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process. J. Clin. Med. 2019, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M. Low-temperature preparation of titania nanorods through direct oxidation of titanium with hydrogen peroxide. J. Cryst. Growth 2004, 269, 347–355. [Google Scholar] [CrossRef]
- Radtke, A.; Bal, M.; Jędrzejewski, T. Novel Titania Nanocoatings Produced by Anodic Oxidation with the Use of Cyclically Changing Potential: Their Photocatalytic Activity and Biocompatibility. Nanomaterials 2018, 8, 712. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Piszczek, P. Bioactivity Studies on Titania Coatings and the Estimation of Their Usefulness in the Modification of Implant Surfaces. Nanomaterials 2017, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania nanotubes for orchestrating osteogenesis at the bone–implant interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Srikant, P.V.S.; Manna, I.; Chatterjee, U.K.; Majumdar, J.D. Chemical oxidation of Ti–6Al–4V for improved wear and corrosion resistance. Surf. Eng. 2008, 24, 442–446. [Google Scholar] [CrossRef]
- Variola, F.; Lauria, A.; Nanci, A.; Rosei, F. Influence of Treatment Conditions on the Chemical Oxidative Activity of H2SO4/H2O2 Mixtures for Modulating the Topography of Titanium. Adv. Eng. Mater. 2009, 11. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef]
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J. Biomed. Mater. Res. 1998, 40, 324–335. [Google Scholar] [CrossRef]
- Radtke, A. 1D Titania Nanoarchitecture as Bioactive and Photoactive Coatings for Modern Implants: A Review. In Application of Titanium Dioxide; Magdalena, J., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Xiao, F.; Tsuru, K.; Hayakawa, S.; Osaka, A. In vitro apatite deposition on titania film derived from chemical treatment of Ti substrates with an oxysulfate solution containing hydrogen peroxide at low temperature. Thin Solid Films 2003, 441, 271–276. [Google Scholar] [CrossRef]
- Walivaara, B.; Aronsson, B.O.; Rodahl, M.; Lausmma, J.; Tengvall, P. Titanium with different oxides: In vitro studies of protein adsorption and contact activation. Biomaterials 1994, 15, 827–834. [Google Scholar] [CrossRef]
- Martino, S.; D’Angelo, F.; Armentano, I.; Kenny, J.M.; Orlacchio, A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012, 30, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Cowden, K.; Dias-Netipanyj, M.F.; Popat, K.C. Adhesion and Proliferation of Human Adipose-Derived Stem Cells on Titania Nanotube Surfaces. Regen. Eng. Transl. Med. 2019, 1–11. [Google Scholar] [CrossRef]
- Ciuffi, S.; Zonefrati, R.; Brandi, M.L. Adipose stem cells for bone tissue repair. Clin. Cases Miner. Bone Metab. 2017, 14, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, M.; Ceccarelli, G.; Cristofaro, F.; Balli, M.; Bertoglio, F.; Bruni, G.; Benedetti, L.; Avanzini, M.A.; Imbriani, M.; Visai, L. Nanostructured TiO2 Surfaces Promote Human Bone Marrow Mesenchymal Stem Cells Differentiation to Osteoblasts. Nanomaterials 2016, 6, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.W.; Zara, J.N.; Zhang, X.; Askarinam, A.; Goyal, R.; Chiang, M.; Yuan, W.; Chang, L.; Corselli, M.; Shen, J.; et al. Perivascular stem cells: A prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl. Med. 2012, 1, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. Rep. 2011, 7, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adiposed-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Sato, M.; Masuoka, K.; Ishihara, M.; Kikuchi, T.; Matsui, T.; Takase, B.; Ishizuka, T.; Kikuchi, M.; Fujikawa, K.; et al. Osteogenic potential of human adipose tissue derived stromal cells as an alternative stem cell source. Cells Tissues Organs 2004, 178, 2–12. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cell from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewallen, E.A.; Jones, D.L.; Dudakovic, A.; Thaler, R.; Paradise, C.R.; Kremers, H.M.; Abdel, M.P.; Kakar, S.; Dietz, A.B.; Cohene, R.C.; et al. Osteogenic potential of human adipose-tissue-derived mesenchymal stromal cells cultured on 3D-printed porous structured titanium. Gene 2016, 581, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safford, K.M.; Safford, S.D.; Gimble, J.M.; Shetty, A.K.; Rice, H.E. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp. Neurol. 2004, 187, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Goulart, V.; Ferreira, L.B.; Duarte, C.A.; Lemos de Lima, I.; Ferreira, E.R.; Candido de Oliveira, B.; Vargas, L.N.; Dotto de Moraes, D.; Silva, I.B.B.; de Oliveira Faria, R.; et al. Mesenchymal stem cells from human adipose tissue and bone repair: A literature review. Biotechnol. Res. Innov. 2018, 2, 74–80. [Google Scholar] [CrossRef]
- Gastaldi, G.; Asti, A.; Scaffino, M.F.; Visai, L.; Saino, E.; Cometa, A.M.; Benazzo, F. Human adipose-derived stem cells (hASCs) proliferate and differentiatein osteoblast-like cells on trabecular titanium scaffolds. J. Biomed. Mater. Res. Part A 2010, 94, 790–799. [Google Scholar]
- Rozila, I.; Azari, P.; Munirah, S.; Wan Safwani, W.K.; Gan, S.N.; Nur Azurah, A.G.; Jahendran, J.; Pingguan-Murphy, B.; Chua, K.H. Differential osteogenic potential of human adipose-derived stem cells co-cultured with human osteoblasts on polymeric microfiber scaffolds. J. Biomed. Mater. Res. Part A 2016, 104, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Zellner, J.; Mueller, M.; Berner, A.; Dienstknecht, T.; Kujat, R.; Nerlich, M.; Hennemann, B.; Koller, M.; Prantl, L.; Angele, M.; et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J. Biomed. Mater. Res. Part A 2010, 94, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Jang, K.A.; Sung, J.H. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol. Surg. 2008, 34, 1323–1326. [Google Scholar] [PubMed]
- Shen, X.; Du, Y.; Shen, W.; Xue, B.; Zhao, Y. Adipose-derived stem cells promote human dermal fibroblast function and increase senescence-associated β-galactosidase mRNA expression through paracrine effects. Mol. Med. Rep. 2014, 10, 3068–3072. [Google Scholar] [CrossRef] [PubMed]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Kasetsart J. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Park, J.Y.; Lee, I.H. Characterization and Morphology of Prepared Titanium Dioxide Nanofibers by Electrospinning. J. Nanosci. Nanotechnol. 2010, 10, 3402–3405. [Google Scholar] [CrossRef] [PubMed]
- Aiempanakit, M.; Helmersson, U.; Aijaz, A.; Larsson, P.; Magnusson, R.; Jensen, J.; Kubart, T. Effect of peak power in reactive high power impulse magnetron sputtering of titanium dioxide. Surf. Coat. Technol. 2011, 205, 4828–4831. [Google Scholar] [CrossRef] [Green Version]
- Sarma, B.K.; Pal, A.R.; Bailung, H.; Chutia, J. Growth of nanocrystalline TiO2 thin films and crystal anisotropy of anatase phase deposited by direct current reactive magnetron sputtering. Mater. Chem. Phys. 2013, 139, 979–987. [Google Scholar] [CrossRef]
- Jing, F.J.; Yukimura, K.; Kato, H.; Lei, Y.F.; You, T.X.; Leng, Y.X.; Huang, N. Film characterization of titanium oxide films prepared by high-power impulse magnetron sputtering. Surf. Coat. Technol. 2011, 206, 967–971. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. Design criteria for wear-resistant nanostructured and glassy-metal coatings. Surf. Coat. Technol. 2004, 177, 317–324. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y.; Rajabi, A. Effects of TiO2 on microstructural, mechanical properties and in-vitro bioactivity of plasma sprayed yttria stabilised zirconia coatings for dental application. Ceram. Int. 2017, 44, 4271–4281. [Google Scholar] [CrossRef]
- Beake, B.D.; Vishnyakov, V.M.; Valizadeh, R.; Colligon, J.S. Influence of mechanical properties on the nanoscratch behaviour of hard nanocomposite TiN/Si3N4 coatings on Si. J. Phys. D Appl. Phys. 2006, 39, 1392–1397. [Google Scholar] [CrossRef]
- Wu, Y.; Zitelli, J.P.; TenHuisen, K.S.; Yu, X.; Libera, M.R. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials 2011, 32, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhong, X.; Wu, X.; Yuan, L.; Zhao, Z.; Wang, H.; Xia, Y.; Feng, Y.; He, J.; Chen, W. The effect of surface roughness and wettability of nanostructured TiO2 film on TCA-8113 epithelial-like cells. Surf. Coat. Technol. 2006, 200, 6155–6160. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Majkowska-Marzec, B.; Strugala, G. Effects of solution composition and electrophoretic deposition voltage on various properties of nanohydroxyapatite coatings on the Ti13Zr13Nb alloy. Ceram. Int. 2018, 44, 19236–19246. [Google Scholar] [CrossRef]
- Sarraf, M.; Razak, B.A.; Nasiri-Tabrizi, B.; Dabbagh, A.; Kasim, N.H.A.; Basirun, W.J.; Bin Sulaiman, E. Nanomechanical properties, wear resistance and in-vitro characterization of Ta2O5 nanotubes coating on biomedical grade Ti–6Al–4V. J. Mech. Behav. Biomed. Mater. 2017, 66, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Booth, F.; Garrido, L.; Aglietti, E.; Pena, P.; Baudín, C. Young’s modulus and hardness of multiphase CaZrO3-MgO ceramics by micro and nanoindentation. J. Eur. Ceram. Soc. 2017, 38, 2194–2201. [Google Scholar] [CrossRef]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kaabi Falahieh Asl, S.; Nemeth, S.; Tan, M.J. Electrophoretic deposition of hydroxyapatite coatings on AZ31 magnesium substrate for biodegradable implant applications. Prog. Cryst. Growth Charact. Mater. 2014, 60, 74–79. [Google Scholar] [CrossRef]
- Matsugi, K.; Endo, T.; Choi, Y.B.; Sasaki, G. Alloy design of Ti alloys using ubiquitous alloying elements and characteristics of their levitation-melted alloys. Mater. Trans. 2010, 51, 740–748. [Google Scholar] [CrossRef] [Green Version]
- Karimzadeh, A.; Ayatollahi, M.R.; Bushroa, A.R.; Herliansyah, M.K. Effect of sintering temperature on mechanical and tribological properties of hydroxyapatite measured by nanoindentation and nanoscratch experiments. Ceram. Int. 2014, 40, 9159–9164. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Jazdzewska, M.; Głodowska, J.; Kalka, P. Effects of electrophoretic deposition times and nanotubular oxide surfaces on properties of the nanohydroxyapatite/nanocopper coating on the Ti13Zr13Nb alloy. Ceram. Int. 2019, 45, 20002–20010. [Google Scholar] [CrossRef]
- Cedillo-Gonzalez, E.I.; Montorosi, M.; Mugoni, C.; Montorosi, M.; Siligardi, C. Improvement of the adhesion between TiO2 nanofilm and glass substrate by roughness modifications. Phys. Procedia 2013, 40, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Manoj Kumar, R.; Kuntal, K.K.; Singh, S.; Gupta, P.; Bhushan, B.; Gopinath, P.; Lahiri, D. Electrophoretic deposition of hydroxyapatite coating on Mg-3Zn alloy for orthopedic application. Surf. Coat. Technol. 2016, 287, 82–92. [Google Scholar] [CrossRef]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Burmester, A.; Luthringer, B.; Willumeit, R.; Feyerabend, F. Comparison of the reaction of bone-derived cells to enhanced MgCl2-salt concentrations. Biomatter 2014, 4, e967616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrivikraman, G.; Madras, G.; Basu, B. In vitro/In vivo assessment and mechanisms of toxicity of bioceramic materials and its wear particulates. RSC Adv. 2014, 4, 12763–12781. [Google Scholar] [CrossRef] [Green Version]
- Bressan, E.; Botticelli, D.; Sivolella, S.; Bengazi, F.; Guazzo, R.; Sbricoli, L.; Ricci, S.; Ferroni, L.; Gardin, C.; Velez, J.U.; et al. Adipose-derived stem cells as a tool for dental implant osseointegration: An experimental study in the dog. Int. J. Mol. Cell. Med. 2015, 4, 197–208. [Google Scholar] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershovich, J.G.; Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Enhanced osteogenesis in cocultures with human mesenchymal stem cells and endothelial cells on polymeric microfiber scaffolds. Tissue Eng. Part A 2013, 19, 2565–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birmingham, E.; Niebur, G.L.; McHugh, P.E.; Shaw, G.; Barry, F.P.; McNamara, L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cells Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Hubka, K.M.; Dahlin, R.L.; Meretoja, V.V.; Kasper, F.K.; Mikos, A.G. Enhancing chondrogenic phenotype for cartilage tissue engineering: Monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng. Part B Rev. 2014, 20, 641–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating nanoscale titania structure with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol. Sci. 2006, 92, 174–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, M.; Pripatnanont, P.; Suttapreyasri, S.; Monmaturapoj, N. In vitro biocompatibility analysis of novel nano-biphasic calcium phosphate scaffolds in different composition ratios. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]







| Biomaterial Sample | Hardness H (GPa) | Young’s Modulus E (GPa) | Maximum Depth of Indentation (nm) | H/E (−) | H3/E2 (GPa) |
|---|---|---|---|---|---|
| Ti6Al4V | 10.94 ± 1.42 | 212.48 ± 16.69 | 472.25 ± 26.11 | 0.0513 ± 0.0034 | 0.0294 ± 0.0078 |
| TNF4S | 6.68 ± 2.59 | 170.85 ± 58.26 | 604.76 ± 85.71 | 0.0388 ± 0.0039 | 0.0105 ± 0.0057 |
| TNF6S | 3.92 ± 1.73 | 133.85 ± 42.12 | 800.94 ± 191.73 | 0.0285 ± 0.0068 | 0.0039 ± 0.0033 |
| TNF10S | 4.59 ± 1.41 | 136.54 ± 27.81 | 718.59 ± 112.31 | 0.0330 ± 0.0047 | 0.0054 ± 0.0028 |
| TNF4C | 5.43 ± 2.15 | 166.34 ± 51.06 | 669.96 ± 127.95 | 0.0322 ± 0.0056 | 0.0063 ± 0.0044 |
| TNF6C | 6.00 ± 2.00 | 165.11 ± 39.18 | 634.01 ± 101.29 | 0.0356 ± 0.0046 | 0.0083 ± 0.0048 |
| TNF10C | 4.69 ± 1.50 | 133.22 ± 20.80 | 709.13 ± 88.34 | 0.0348 ± 0.0054 | 0.0064 ± 0.0050 |
| TNF72a | 6.27 ± 0.88 | 170.92 ± 15.73 | 601.98 ± 41.46 | 0.0356 ± 0.0021 | 0.0085 ± 0.0021 |
| TNF72b | 7.68 ± 1.78 | 180.18 ± 27.40 | 561.09 ± 80.11 | 0.0421 ± 0.0044 | 0.0143 ± 0.0053 |
| Biomaterial Sample | Nanoscratch-Test Properties | |
|---|---|---|
| Critical Force (mN) | Critical Friction Force (mN) | |
| TNF4S | 164.20 ± 61.12 | 131.68 ± 52.66 |
| TNF6S | 107.40 ± 27.27 | 91.86 ± 22.80 |
| TNF10S | 116.69 ± 28.67 | 90.69 ± 21.53 |
| TNF4C | 130.95 ± 47.15 | 104.83 ± 47.34 |
| TNF6C | 139.03 ± 34.59 | 105.91 ± 32.16 |
| TNF10C | 140.91 ± 34.10 | 117.33 ± 42.05 |
| TNF72a | 203.91 ± 37.59 | 183.24 ± 61.29 |
| TNF72b | 205.15 ± 48.96 | 140.27 ± 46.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlert, M.; Roszek, K.; Jędrzejewski, T.; Bartmański, M.; Radtke, A. Titania Nanofiber Scaffolds with Enhanced Biointegration Activity—Preliminary In Vitro Studies. Int. J. Mol. Sci. 2019, 20, 5642. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225642
Ehlert M, Roszek K, Jędrzejewski T, Bartmański M, Radtke A. Titania Nanofiber Scaffolds with Enhanced Biointegration Activity—Preliminary In Vitro Studies. International Journal of Molecular Sciences. 2019; 20(22):5642. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225642
Chicago/Turabian StyleEhlert, Michalina, Katarzyna Roszek, Tomasz Jędrzejewski, Michał Bartmański, and Aleksandra Radtke. 2019. "Titania Nanofiber Scaffolds with Enhanced Biointegration Activity—Preliminary In Vitro Studies" International Journal of Molecular Sciences 20, no. 22: 5642. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225642