Innovative Biomaterials for Bone Regrowth
Abstract
:1. Introduction
2. Bone Biology
3. Bone Fracture and Diseases
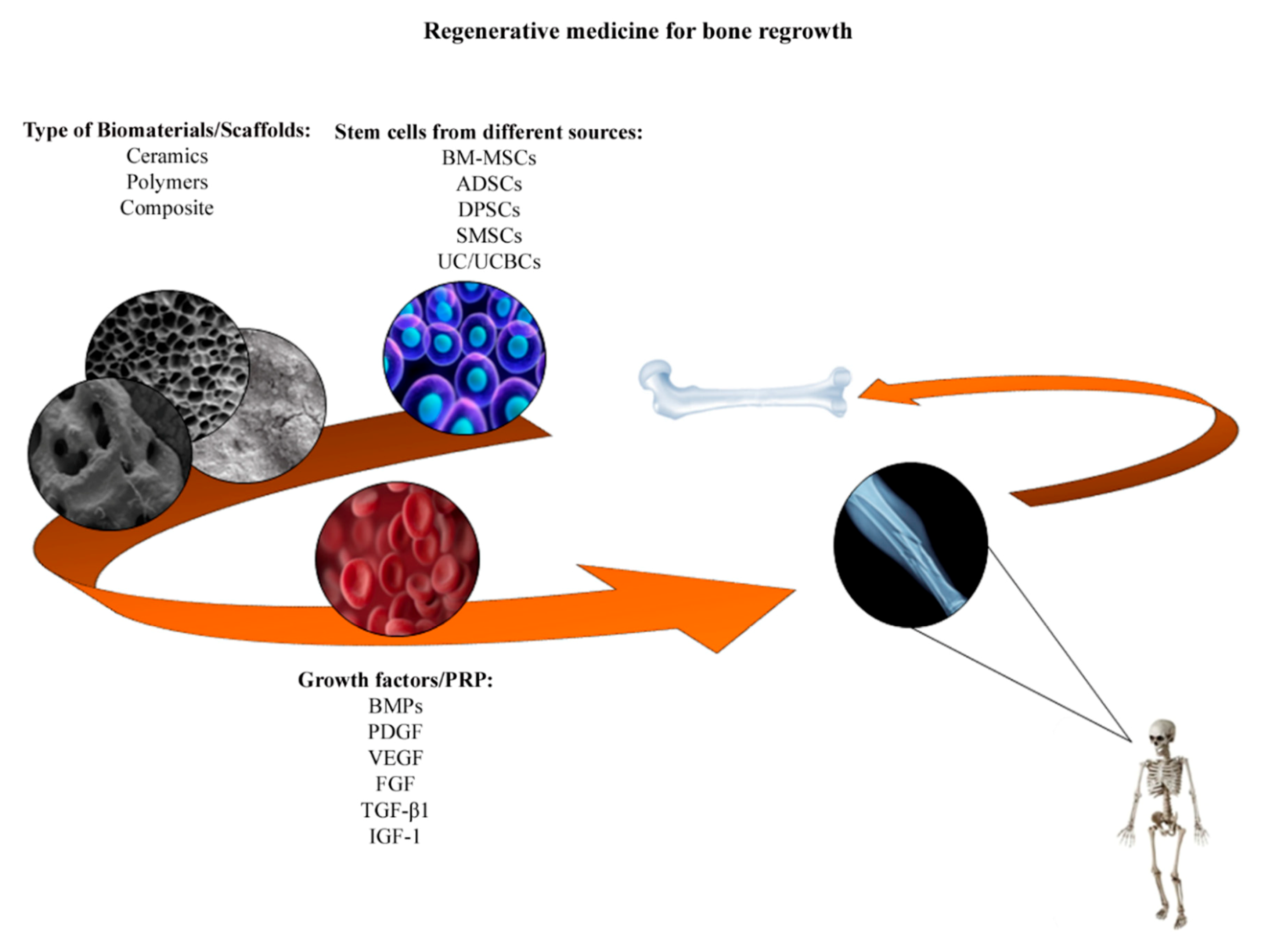
4. Tissue Engineering: Stem Cells and Biomaterials in Bone Formation
5. Mesenchymal Stem Cells
6. Biomaterials
7. Ceramic Biomaterials
8. Polymers
9. Composite Biomaterials
10. Growth Factors and Platelet-Rich Plasma (PRP)
11. Conclusions
Funding
Conflicts of Interest
References
- Martin, V.; Bettencourt, A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mat. Sci. Eng. C-Mater. 2018, 82, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Ferreira, J.M.F.; Andronescu, E.; Ficai, D.; Sonmez, M.; Ficai, A. Multifunctional materials for bone cancer treatment. Int. J. Nanomed. 2014, 9, 2713–2725. [Google Scholar]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S.; Sandri, M.; Logroscino, G. Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomater. 2007, 3, 961–969. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Tsuchiya, A.; Hayashi, K.; Tsuru, K.; Ohe, G. Physical and Histological Comparison of Hydroxyapatite, Carbonate Apatite, and beta-Tricalcium Phosphate Bone Substitutes. Materials 2018, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.S.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Kanno, T.; Tatsumi, H.; Miyamoto, K.; Sha, J.; Hideshima, K.; Matsuzaki, Y. Feasibility of a Three-Dimensional Porous Uncalcined and Unsintered Hydroxyapatite/poly-d/l-lactide Composite as a Regenerative Biomaterial in Maxillofacial Surgery. Materials 2018, 11, 2047. [Google Scholar] [CrossRef] [PubMed]
- Brodano, G.B.; Mazzoni, E.; Tognon, M.; Griffoni, C.; Manfrini, M. Human mesenchymal stem cells and biomaterials interaction: A promising synergy to improve spine fusion. Eur. Spine J. 2012, 21, S3–S9. [Google Scholar] [CrossRef]
- Bhuiyan, D.B.; Middleton, J.C.; Tannenbaum, R.; Wick, T.M. Bone regeneration from human mesenchymal stem cells on porous hydroxyapatite-PLGA-collagen bioactive polymer scaffolds. Bio-Med. Mater. Eng. 2017, 28, 671–685. [Google Scholar] [CrossRef]
- Pulyala, P.; Singh, A.; Dias-Netipanyj, M.F.; Cogo, S.C.; Santos, L.S.; Soares, P.; Gopal, V.; Suganthan, V.; Manivasagam, G.; Popat, K.C. In-vitro cell adhesion and proliferation of adipose derived stem cell on hydroxyapatite composite surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1305–1316. [Google Scholar] [CrossRef]
- Mazzoni, E.; D’Agostino, A.; Manfrini, M.; Maniero, S.; Puozzo, A.; Bassi, E.; Marsico, S.; Fortini, C.; Trevisiol, L.; Patergnani, S.; et al. Human adipose stem cells induced to osteogenic differentiation by an innovative collagen/hydroxylapatite hybrid scaffold. FASEB J. 2017, 31, 4555–4565. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Glimcher, M.J.; Cooper, R.R.; Recker, R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr. Course Lect. 1996, 45, 371–386. [Google Scholar] [PubMed]
- Downey, P.A.; Siegel, M.I. Bone biology and the clinical implications for osteoporosis. Phys. Ther. 2006, 86, 77–91. [Google Scholar] [CrossRef]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsenty, G. Transcriptional control of skeletogenesis. Annu. Rev. Genom. Hum. Genet. 2008, 9, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Oates, T.; Garlet, G.P. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011, 3, 5304. [Google Scholar] [CrossRef]
- Jilka, R.L.; O’Brien, C.A. The Role of Osteocytes in Age-Related Bone Loss. Curr. Osteoporos. Rep. 2016, 14, 16–25. [Google Scholar] [CrossRef]
- Schett, G.; Teitelbaum, S.L. Osteoclasts and arthritis. J. Bone Miner. Res. 2009, 24, 1142–1146. [Google Scholar] [CrossRef]
- Miller, S.C.; de Saint-Georges, L.; Bowman, B.M.; Jee, W.S. Bone lining cells: Structure and function. Scanning Microsc. 1989, 3, 953–960. [Google Scholar] [PubMed]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar] [PubMed]
- Schett, G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Why should many skeletal scientists and clinicians learn the Utah paradigm of skeletal physiology? J. Musculoskelet. Neuronal Interact. 2001, 2, 121–130. [Google Scholar] [PubMed]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell ... and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Sobacchi, C.; Schulz, A.; Coxon, F.P.; Villa, A.; Helfrich, M.H. Osteopetrosis: Genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 2013, 9, 522–536. [Google Scholar] [CrossRef]
- Habibovic, P. (*) Strategic Directions in Osteoinduction and Biomimetics. Tissue Eng. Part. A 2017, 23, 1295–1296. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Oral, A.; Kucukdeveci, A.A.; Varela, E.; Ilieva, E.M.; Valero, R.; Berteanu, M.; Christodoulou, N. Osteoporosis. The role of physical and rehabilitation medicine physicians. The European perspective based on the best evidence. A paper by the UEMS-PRM Section Professional Practice Committee. Eur. J. Phys. Rehabil. Med. 2013, 49, 565–577. [Google Scholar] [PubMed]
- Wright, N.C.; Hooker, E.R.; Nielson, C.M.; Ensrud, K.E.; Harrison, S.L.; Orwoll, E.S.; Barrett-Connor, E.; Osteoporotic Fractures in Men (MrOS) Study Research Group. The epidemiology of wrist fractures in older men: The Osteoporotic Fractures in Men (MrOS) study. Osteoporos. Int. 2018, 29, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Henss, A.; Rohnke, M.; Gelinsky, M. A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.M.Y.; Wong, H.; Zhang, N.; Chow, S.K.H.; Chau, W.W.; Wang, J.; Chim, Y.N.; Leung, K.S.; Cheung, W.H. The relationship between sarcopenia and fragility fracture-a systematic review. Osteoporos. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, D.; Cawthon, P.M.; Ensrud, K.E.; Stefanick, M.L.; Kado, D.M.; Boudreau, R.; Greenspan, S.; Newman, A.B.; Zmuda, J.; Orwoll, E.S.; et al. Risk of Nonspine Fractures in Older Adults with Sarcopenia, Low Bone Mass, or Both. J. Am. Geriatr. Soc. 2015, 63, 1733–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binkley, N.; Buehring, B. Beyond FRAX: it’s time to consider “sarco-osteopenia”. J. Clin. Densitom. 2009, 12, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Borghi, A.; Selvatici, R.; Mazzoni, E.; Bononi, I.; Corazza, M.; Kussini, J.; Montinari, E.; Gafa, R.; Tognon, M.; et al. Association of Retinoic Acid Receptor beta Gene with Onset and Progression of Lichen Sclerosus-Associated Vulvar Squamous Cell Carcinoma. JAMA Dermatol. 2018, 154, 819–823. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Borghi, A.; Selvatici, R.; Magri, E.; Bianchini, E.; Montinari, E.; Corazza, M.; Virgili, A.; Tognon, M.; Martini, F. Hypermethylation-Induced Inactivation of the IRF6 Gene as a Possible Early Event in Progression of Vulvar Squamous Cell Carcinoma Associated with Lichen Sclerosus. JAMA Dermatol. 2016, 152, 928–932. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Bononi, I.; Puozzo, A.; Govoni, M.; Foschi, V.; Lanza, G.; Gafa, R.; Gaboriaud, P.; Touze, F.A.; Selvatici, R.; et al. Merkel Cell Carcinomas Arising in Autoimmune Disease Affected Patients Treated with Biologic Drugs, Including Anti-TNF. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 3929–3934. [Google Scholar] [CrossRef] [Green Version]
- Tognon, M.; Luppi, M.; Corallini, A.; Taronna, A.; Barozzi, P.; Rotondo, J.C.; Comar, M.; Casali, M.V.; Bovenzi, M.; D’Agostino, A.; et al. Immunologic evidence of a strong association between non-Hodgkin lymphoma and simian virus 40. Cancer 2015, 121, 2618–2626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [PubMed]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Gerrand, C.; Athanasou, N.; Brennan, B.; Grimer, R.; Judson, I.; Morland, B.; Peake, D.; Seddon, B.; Whelan, J. UK guidelines for the management of bone sarcomas. Clin. Sarcoma Res. 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.; Kempf-Bielack, B.; Von Kalle, T.; Schwarz, R.; Wirth, T.; Kager, L.; Whelan, J. Controversies in childhood osteosarcoma. Minerva Pediatrica 2013, 65, 125–148. [Google Scholar] [PubMed]
- Kundu, Z.S. Classification, imaging, biopsy and staging of osteosarcoma. Indian J. Orthop. 2014, 48, 238–246. [Google Scholar] [PubMed]
- Biazzo, A.; De Paolis, M. Multidisciplinary approach to osteosarcoma. Acta Orthop. Belg. 2016, 82, 690–698. [Google Scholar] [PubMed]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. Sicot-J. 2018, 4, 12. [Google Scholar] [CrossRef]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer 2018, 18, 39–50. [Google Scholar] [CrossRef]
- Meyers, P.A. Systemic therapy for osteosarcoma and Ewing sarcoma. In American Society of Clinical Oncology Educational Book, Proceedings of the American Society of Clinical Oncology Annual Meeting, Chicago, IL, USA, 29 May–2 June 2015; American Society of Clinical Oncology: Alexandria, VA, USA; pp. 644–647.
- Anninga, J.K.; Gelderblom, H.; Fiocco, M.; Kroep, J.R.; Taminiau, A.H.; Hogendoorn, P.C.; Egeler, R.M. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand? Eur. J. Cancer 2011, 47, 2431–2445. [Google Scholar] [CrossRef]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Hess, U.; Shahabi, S.; Treccani, L.; Streckbein, P.; Heiss, C.; Rezwan, K. Co-delivery of cisplatin and doxorubicin from calcium phosphate beads/matrix scaffolds for osteosarcoma therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yu, S.; Li, D.; Gong, Y.; Zang, J.; Liu, J.; Chen, X. The effect of PLGA-based hydrogel scaffold for improving the drug maximum-tolerated dose for in situ osteosarcoma treatment. Colloids Surf. B Biointerfaces 2018, 172, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Manfrini, M.; Di Bona, C.; Canella, A.; Lucarelli, E.; Pellati, A.; D’Agostino, A.; Barbanti-Brodano, G.; Tognon, M. Mesenchymal stem cells from patients to assay bone graft substitutes. J. Cell. Physiol. 2013, 228, 1229–1237. [Google Scholar] [CrossRef]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef] [PubMed]
- Verboket, R.; Leiblein, M.; Seebach, C.; Nau, C.; Janko, M.; Bellen, M.; Bonig, H.; Henrich, D.; Marzi, I. Autologous cell-based therapy for treatment of large bone defects: From bench to bedside. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2018, 44, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.; Hernigou, P.; Zilkens, C.; Herten, M.; Li, X.; Fischer, J.; Krauspe, R. Cell therapy in bone healing disorders. Orthop. Rev. 2010, 2, e20. [Google Scholar] [CrossRef] [PubMed]
- Noth, U.; Reichert, J.; Reppenhagen, S.; Steinert, A.; Rackwitz, L.; Eulert, J.; Beckmann, J.; Tingart, M. Cell based therapy for the treatment of femoral head necrosis. Der Orthop. 2007, 36, 466–471. [Google Scholar]
- Seebach, C.; Henrich, D.; Meier, S.; Nau, C.; Bonig, H.; Marzi, I. Safety and feasibility of cell-based therapy of autologous bone marrow-derived mononuclear cells in plate-stabilized proximal humeral fractures in humans. J. Transl. Med. 2016, 14, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sponer, P.; Filip, S.; Kucera, T.; Brtkova, J.; Urban, K.; Palicka, V.; Koci, Z.; Syka, M.; Bezrouk, A.; Sykova, E. Utilizing Autologous Multipotent Mesenchymal Stromal Cells and beta-Tricalcium Phosphate Scaffold in Human Bone Defects: A Prospective, Controlled Feasibility Trial. Biomed. Res. Int. 2016, 2016, 2076061. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.H.; Liu, S.W.; Xiong, L.; Qiu, P.; Ding, L.H.; Xiong, S.L.; Li, J.T.; Liao, X.G.; Tang, Z.M. Scaffolds for the repair of bone defects in clinical studies: A systematic review. J. Orthop. Surg. Res. 2018, 13, 33. [Google Scholar] [CrossRef]
- Jia, Z.; Liang, Y.; Xu, X.; Li, X.; Liu, Q.; Ou, Y.; Duan, L.; Zhu, W.; Lu, W.; Xiong, J.; et al. Isolation and characterization of human mesenchymal stem cells derived from synovial fluid by magnetic-activated cell sorting (MACS). Cell Biol. Int. 2018, 42, 262–271. [Google Scholar] [CrossRef]
- Schneider, S.; Unger, M.; van Griensven, M.; Balmayor, E.R. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur. J. Med. Res. 2017, 22, 17. [Google Scholar] [CrossRef]
- Stanko, P.; Altanerova, U.; Jakubechova, J.; Repiska, V.; Altaner, C. Dental Mesenchymal Stem/Stromal Cells and Their Exosomes. Stem Cells Int. 2018, 2018, 8973613. [Google Scholar] [CrossRef]
- Abbaspanah, B.; Momeni, M.; Ebrahimi, M.; Mousavi, S.H. Advances in perinatal stem cells research: A precious cell source for clinical applications. Regen. Med. 2018, 13, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, A.; Uchida, S.; Utsunomiya, H.; Tsukamoto, M.; Nakashima, H.; Nakamura, E.; Pascual-Garrido, C.; Sekiya, I.; Sakai, A. Isolation and Characterization of Synovial Mesenchymal Stem Cell Derived from Hip Joints: A Comparative Analysis with a Matched Control Knee Group. Stem Cells Int. 2017, 2017, 9312329. [Google Scholar] [CrossRef] [PubMed]
- Mennan, C.; Wright, K.; Bhattacharjee, A.; Balain, B.; Richardson, J.; Roberts, S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed. Res. Int. 2013, 2013, 916136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An In Vitro Comparative Study of Multisource Derived Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Yamada, Y.; Nakamura, S.; Ueda, M. Osteogenic Potential of Effective Bone Engineering Using Dental Pulp Stem Cells, Bone Marrow Stem Cells, and Periosteal Cells for Osseointegration of Dental Implants. Int. J. Oral Max. Impl. 2011, 26, 947–954. [Google Scholar]
- Obermeyer, T.S.; Yonick, D.; Lauing, K.; Stock, S.R.; Nauer, R.; Strotman, P.; Shankar, R.; Gamelli, R.; Stover, M.; Callaci, J.J. Mesenchymal stem cells facilitate fracture repair in an alcohol-induced impaired healing model. J. Orthop. Trauma 2012, 26, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Mebarki, M.; Coquelin, L.; Layrolle, P.; Battaglia, S.; Tossou, M.; Hernigou, P.; Rouard, H.; Chevallier, N. Enhanced human bone marrow mesenchymal stromal cell adhesion on scaffolds promotes cell survival and bone formation. Acta Biomater. 2017, 59, 94–107. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Vranceanu, M.D.; Antoniac, I.; Miculescu, F.; Saban, R. The influence of the ceramic phase on the porosity of some biocomposites with collagen matrix used as bone substitutes. J. Optoelectron. Adv. Mater. 2012, 14, 671–677. [Google Scholar]
- Liu, G.; Li, Y.; Sun, J.; Zhou, H.; Zhang, W.; Cui, L.; Cao, Y. In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood-derived mesenchymal stem cells on partially demineralized bone matrix. Tissue Eng. Part A 2010, 16, 971–982. [Google Scholar] [CrossRef]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication, and apatite formation. J. Biomed. Mater. Res. Part. A 2014, 102, 254–274. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. 3D printed tricalcium phosphate scaffolds: Effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater. Sci. 2013, 1, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Montesi, M.; Panseri, S.; Dapporto, M.; Tampieri, A.; Sprio, S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PLoS ONE 2017, 12, e0172100. [Google Scholar] [CrossRef] [PubMed]
- Sprio, S.; Dapporto, M.; Montesi, M.; Panseri, S.; Lattanzi, W.; Pola, E.; Logroscino, G.; Tampieri, A. Novel Osteointegrative Sr-Substituted Apatitic Cements Enriched with Alginate. Materials 2016, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, H.L. Calcium Phosphate Scaffolds Combined with Bone Morphogenetic Proteins or Mesenchymal Stem Cells in Bone Tissue Engineering. Chin. Med. J.-Peking 2015, 128, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.A.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Russo, A.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Magnetic Hydroxyapatite Bone Substitutes to Enhance Tissue Regeneration: Evaluation In Vitro Using Osteoblast-Like Cells and In Vivo in a Bone Defect. PLoS ONE 2012, 7, e38710. [Google Scholar] [CrossRef]
- Russo, A.; Bianchi, M.; Sartori, M.; Boi, M.; Giavaresi, G.; Salter, D.M.; Jelic, M.; Maltarello, M.C.; Ortolani, A.; Sprio, S.; et al. Bone regeneration in a rabbit critical femoral defect by means of magnetic hydroxyapatite macroporous scaffolds. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2018, 106, 546–554. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Athirasala, A.; Twohig, C.; Boda, S.K.; Bertassoni, L.E. Biomaterials for Craniofacial Bone Regeneration. Dent. Clin. N. Am. 2017, 61, 835–856. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds based bone tissue engineering: The role of chitosan. Tissue Eng. Part. B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Stegemann, J.P. Thermogelling chitosan and collagen composite hydrogels initiated with beta-glycerophosphate for bone tissue engineering. Biomaterials 2010, 31, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Correlo, V.M.; Sol, P.C.; Bhattacharya, M.; Srouji, S.; Livne, E.; Reis, R.L.; Neves, N.M. Chitosan-poly(butylene succinate) scaffolds and human bone marrow stromal cells induce bone repair in a mouse calvaria model. J. Tissue Eng. Regen. Med. 2012, 6, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sawkins, M.J.; Bowen, W.; Dhadda, P.; Markides, H.; Sidney, L.E.; Taylor, A.J.; Rose, F.R.; Badylak, S.F.; Shakesheff, K.M.; White, L.J. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 2013, 9, 7865–7873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.J.; Grainger, D.W. Demineralized bone matrix as a vehicle for delivering endogenous and exogenous therapeutics in bone repair. Adv. Drug Deliv. Rev. 2012, 64, 1123–1128. [Google Scholar] [CrossRef]
- Dozza, B.; Salamanna, F.; Baleani, M.; Giavaresi, G.; Parrilli, A.; Zani, L.; Lucarelli, E.; Martini, L.; Fini, M.; Donati, D.M. Nonunion fracture healing: Evaluation of effectiveness of demineralized bone matrix and mesenchymal stem cells in a novel sheep bone nonunion model. J. Tissue Eng. Regen. Med. 2018, 12, 1972–1985. [Google Scholar] [CrossRef]
- Desai, P.; Hasan, S.M.; Zambrana, L.; Hegde, V.; Saleh, A.; Cohn, M.R.; Lane, J.M. Bone Mesenchymal Stem Cells with Growth Factors Successfully Treat Nonunions and Delayed Unions. HSS J. Musculoskelet. J. Hosp. Spec. Surg. 2015, 11, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Remya, K.R.; Joseph, J.; Mani, S.; John, A.; Varma, H.K.; Ramesh, P. Nanohydroxyapatite incorporated electrospun polycaprolactone/polycaprolactone-polyethyleneglycol-polycaprolactone blend scaffold for bone tissue engineering applications. J. Biomed. Nanotechnol. 2013, 9, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariepy, E.; Shive, M.; Bichara, A.; Berrada, M.; Le Garrec, D.; Chenite, A.; Leroux, J.C. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2004, 57, 53–63. [Google Scholar] [CrossRef]
- Fan, H.Y.; Dash, A.K. Effect of cross-linking on the in vitro release kinetics of doxorubicin from gelatin un-plants. Int. J. Pharm. 2001, 213, 103–116. [Google Scholar] [CrossRef]
- Niemeyer, P.; Krause, U.; Fellenberg, J.; Kasten, P.; Seckinger, A.; Ho, A.D.; Simank, H.G. Evaluation of mineralized collagen and alpha-tricalcium phosphate as scaffolds for tissue engineering of bone using human mesenchymal stem cells. Cells Tissues Organs 2004, 177, 68–78. [Google Scholar] [CrossRef]
- D’Agostino, A.; Trevisiol, L.; Favero, V.; Gunson, M.J.; Pedica, F.; Nocini, P.F.; Arnett, G.W. Hydroxyapatite/Collagen Composite Is a Reliable Material for Malar Augmentation. J. Oral Maxillofac. Surg. 2016, 74, 1238.e1–1238.e15. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Salvatorelli, L.; Fabbi, C.; Figallo, E.; Gulisano, M.; Parenti, R.; Magro, G.; Colarossi, C.; et al. Bone augmentation after ectopic implantation of a cell-free collagen-hydroxyapatite scaffold in the mouse. Sci. Rep. 2016, 6, 36399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, Q.; Du, B.B.; Cao, L.; Lin, H.; Fan, Z.Y.; Dong, J. Enhanced bone regeneration composite scaffolds of PLLA/beta-TCP matrix grafted with gelatin and HAp. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 60–69. [Google Scholar] [CrossRef]
- Arafat, M.T.; Lam, C.X.; Ekaputra, A.K.; Wong, S.Y.; Li, X.; Gibson, I. Biomimetic composite coating on rapid prototyped scaffolds for bone tissue engineering. Acta Biomater. 2011, 7, 809–820. [Google Scholar] [CrossRef]
- Andronescu, E.; Ficai, A.; Albu, M.G.; Mitran, V.; Sonmez, M.; Ficai, D.; Ion, R.; Cimpean, A. Collagen-hydroxyapatite/cisplatin drug delivery systems for locoregional treatment of bone cancer. Technol. Cancer Res. Treat. 2013, 12, 275–284. [Google Scholar] [CrossRef]
- Ficai, D.; Sonmez, M.; Albu, M.G.; Mihaiescu, D.E.; Ficai, A.; Bleotu, C. Antitumoral materials with regenerative function obtained using a layer-by-layer technique. Drug Des. Dev. 2015, 9, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozano, F.J.; Insausti, C.L.; Meseguer, L.; Ramirez, M.C.; Martinez, S.; Moraleda, J.M. Tissue engineering with dental pulp stem cells: Isolation, characterization, and osteogenic differentiation. J. Craniofacial Surg. 2012, 23, e571–e575. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, H.S.; Pashkuleva, I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kneser, U.; Schaefer, D.J.; Polykandriotis, E.; Horch, R.E. Tissue engineering of bone: The reconstructive surgeon’s point of view. J. Cell. Mol. Med. 2006, 10, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Fischerauer, E.E.; Manninger, M.; Seles, M.; Janezic, G.; Pichler, K.; Ebner, B.; Weinberg, A.M. BMP-6 and BMPR-1a are up-regulated in the growth plate of the fractured tibia. J. Orthop. Res. 2013, 31, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Lee, J.H.; Park, S.; Kim, Y.J.; Seo, J.; Shim, H.E.; Kim, K.S.; Jang, H.S.; Chung, H.M.; Oh, S.G.; et al. Effect of BMP-2 Delivery Mode on Osteogenic Differentiation of Stem Cells. Stem Cells Int. 2017, 2017, 7859184. [Google Scholar] [CrossRef] [PubMed]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef]
- Cahill, K.S.; McCormick, P.C.; Levi, A.D. A comprehensive assessment of the risk of bone morphogenetic protein use in spinal fusion surgery and postoperative cancer diagnosis. J. Neurosurg.-Spine 2015, 23, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecina, M.; Giltaij, L.R.; Vukicevic, S. Orthopaedic applications of osteogenic protein-1 (BMP-7). Int. Orthop. 2001, 25, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccaro, A.R.; Patel, T.; Fischgrund, J.; Anderson, D.G.; Truumees, E.; Herkowitz, H.; Phillips, F.; Hilibrand, A.; Albert, T.J. A pilot safety and efficacy study of OP-1 putty (rhBMP-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur. Spine J. 2003, 12, 495–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccaro, A.R.; Patel, T.; Fischgrund, J.; Anderson, D.G.; Truumees, E.; Herkowitz, H.; Phillips, F.; Hilibrand, A.; Albert, T.J. A 2-year follow-up pilot study evaluating the safety and efficacy of op-1 putty (rhbmp-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur. Spine J. 2005, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Berk, J.; Almojaly, S.A.; Goodloe, S., III; Margarone, J., III; Sullivan, M.; Dziak, R. Effects of platelet-derived growth factor, vitamin D and parathyroid hormone on osteoblasts derived from cancer patients on chronic bisphosphonate therapy. Int. J. Mol. Med. 2009, 23, 407–413. [Google Scholar] [Green Version]
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. 1), 48–54. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.; Mohan, S.; Genasan, K.; Murali, M.R.; Naveen, S.V.; Talebian, S.; McKean, R.; Kamarul, T. Synergistic interaction of platelet derived growth factor (PDGF) with the surface of PLLA/Col/HA and PLLA/HA scaffolds produces rapid osteogenic differentiation. Colloids Surf. B Biointerfaces 2016, 139, 68–78. [Google Scholar] [CrossRef]
- Paglia, D.N.; Singh, H.; Karukonda, T.; Drissi, H.; Moss, I.L. PDGF-BB Delays Degeneration of the Intervertebral Discs in a Rabbit Preclinical Model. Spine 2016, 41, E449–E458. [Google Scholar] [CrossRef]
- DiGiovanni, C.W.; Lin, S.S.; Baumhauer, J.F.; Daniels, T.; Younger, A.; Glazebrook, M.; Anderson, J.; Anderson, R.; Evangelista, P.; Lynch, S.E.; et al. Recombinant human platelet-derived growth factor-BB and beta-tricalcium phosphate (rhPDGF-BB/beta-TCP): An alternative to autogenous bone graft. J. Bone Jt. Surg. Am. 2013, 95, 1184–1192. [Google Scholar] [CrossRef]
- Fennen, M.; Pap, T.; Dankbar, B. Smad-dependent mechanisms of inflammatory bone destruction. Arthritis Res. Ther. 2016, 18, 279. [Google Scholar] [CrossRef]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugjoo, M.B.; Amarpal; Abdelbaset-Ismail, A.; Aithal, H.P.; Kinjavdekar, P.; Pawde, A.M.; Kumar, G.S.; Sharma, G.T. Mesenchymal stem cells with IGF-1 and TGF- beta1 in laminin gel for osteochondral defects in rabbits. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 93, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Farlie, P.; Penington, A.J. Bone regeneration in a rabbit critical-sized skull defect using autologous adipose-derived cells. Tissue Eng. Pt. A 2008, 14, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Murukesh, N.; Dive, C.; Jayson, G.C. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br. J. Cancer 2010, 102, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deckers, M.M.L.; Karperien, M.; van der Bent, C.; Yamashita, T.; Papapoulos, S.E.; Löwik, C.W.G.M. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 2000, 141, 1667–1674. [Google Scholar] [CrossRef]
- Yeh, L.C.; Lee, J.C. Osteogenic protein-1 increases gene expression of vascular endothelial growth factor in primary cultures of fetal rat calvaria cells. Mol. Cell. Endocrinol. 1999, 153, 113–124. [Google Scholar] [CrossRef]
- Goad, D.L.; Rubin, J.; Wang, H.; Tashjian, A.H., Jr.; Patterson, C. Enhanced expression of vascular endothelial growth factor in human SaOS-2 osteoblast-like cells and murine osteoblasts induced by insulin-like growth factor I. Endocrinology 1996, 137, 2262–2268. [Google Scholar] [CrossRef]
- Saadeh, P.B.; Mehrara, B.J.; Steinbrech, D.S.; Spector, J.A.; Greenwald, J.A.; Chin, G.S.; Ueno, H.; Gittes, G.K.; Longaker, M.T. Mechanisms of fibroblast growth factor-2 modulation of vascular endothelial growth factor expression by osteoblastic cells. Endocrinology 2000, 141, 2075–2083. [Google Scholar] [CrossRef]
- Rabie, A.B.; Lu, M. Basic fibroblast growth factor up-regulates the expression of vascular endothelial growth factor during healing of allogeneic bone graft. Arch. Oral Biol. 2004, 49, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthetic Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, K.J.; Bennett, J.; Gronowicz, G.; Adams, D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2005, 63, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Roldan, J.C.; Jepsen, S.; Miller, J.; Freitag, S.; Rueger, D.C.; Acil, Y.; Terheyden, H. Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone 2004, 34, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Shafieian, R.; Matin, M.M.; Rahpeyma, A.; Fazel, A.; Sedigh, H.S.; Nabavi, A.S.; Hassanzadeh, H.; Ebrahimzadeh-Bideskan, A. Effects of Human Adipose-derived Stem Cells and Platelet-Rich Plasma on Healing Response of Canine Alveolar Surgical Bone Defects. Arch. Bone Jt. Surg. 2017, 5, 406–418. [Google Scholar] [PubMed]
- Mooren, R.E.; Dankers, A.C.; Merkx, M.A.; Bronkhorst, E.M.; Jansen, J.A.; Stoelinga, P.J. The effect of platelet-rich plasma on early and late bone healing using a mixture of particulate autogenous cancellous bone and Bio-Oss: An experimental study in goats. Int. J. Oral Maxillofac. Surg. 2010, 39, 371–378. [Google Scholar] [CrossRef]
- Mooren, R.E.; Merkx, M.A.; Bronkhorst, E.M.; Jansen, J.A.; Stoelinga, P.J. The effect of platelet-rich plasma on early and late bone healing: An experimental study in goats. Int. J. Oral Maxillofac. Surg. 2007, 36, 626–631. [Google Scholar] [CrossRef]
- Ranly, D.M.; Lohmann, C.H.; Andreacchio, D.; Boyan, B.D.; Schwartz, Z. Platelet-rich plasma inhibits demineralized bone matrix-induced bone formation in nude mice. J. Bone Jt. Surg. Am. 2007, 89, 139–147. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030618
Iaquinta MR, Mazzoni E, Manfrini M, D’Agostino A, Trevisiol L, Nocini R, Trombelli L, Barbanti-Brodano G, Martini F, Tognon M. Innovative Biomaterials for Bone Regrowth. International Journal of Molecular Sciences. 2019; 20(3):618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030618
Chicago/Turabian StyleIaquinta, Maria Rosa, Elisa Mazzoni, Marco Manfrini, Antonio D’Agostino, Lorenzo Trevisiol, Riccardo Nocini, Leonardo Trombelli, Giovanni Barbanti-Brodano, Fernanda Martini, and Mauro Tognon. 2019. "Innovative Biomaterials for Bone Regrowth" International Journal of Molecular Sciences 20, no. 3: 618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030618




