In Vitro Activity of the Bacteriophage Endolysin HY-133 against Staphylococcus aureus Small-Colony Variants and Their Corresponding Wild Types
Abstract
:1. Introduction
2. Results
2.1. Genotyping by Pulsed-Field Gel Electrophoresis (PFGE)
2.2. Antimicrobial Susceptibility Testing
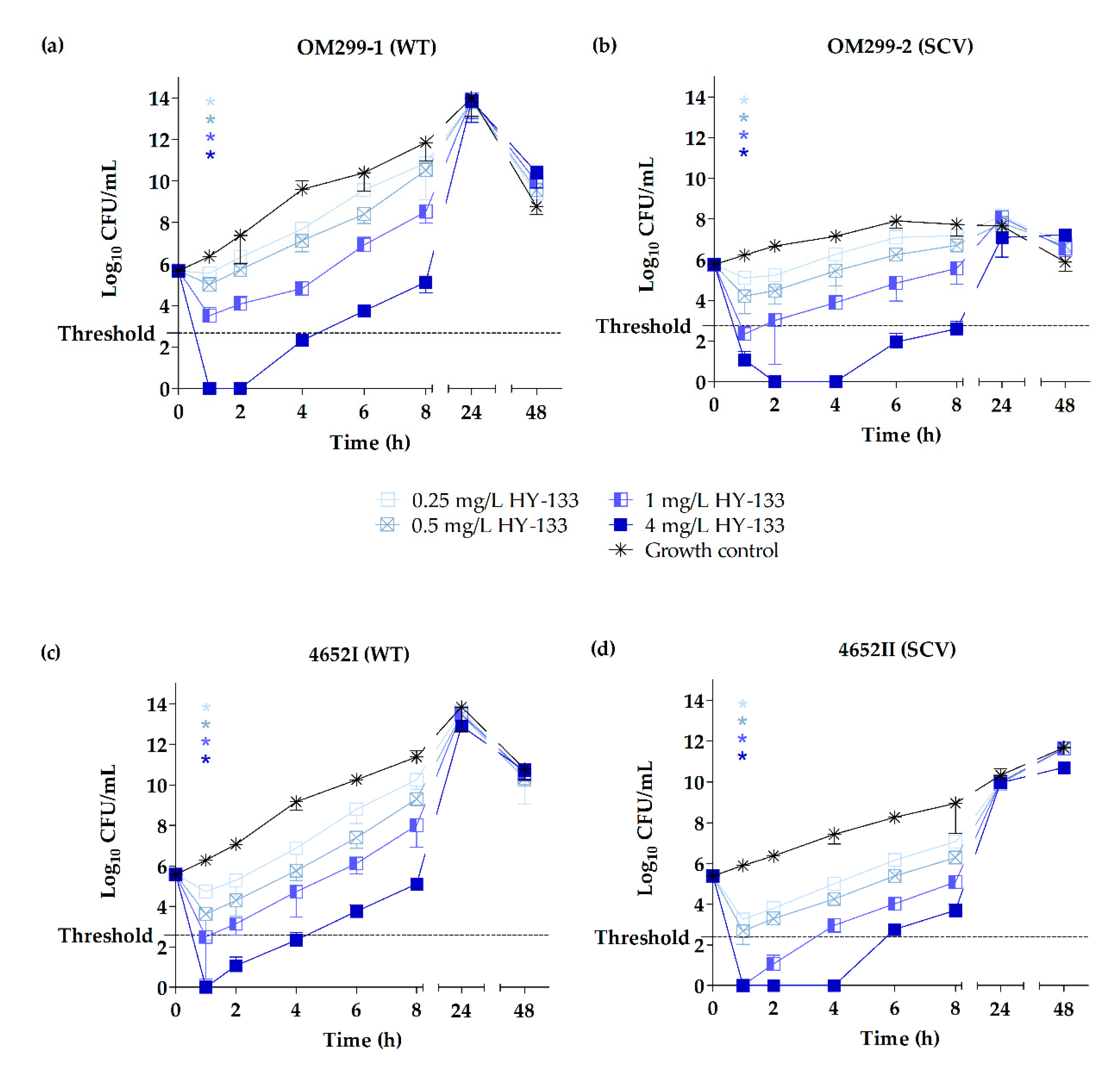
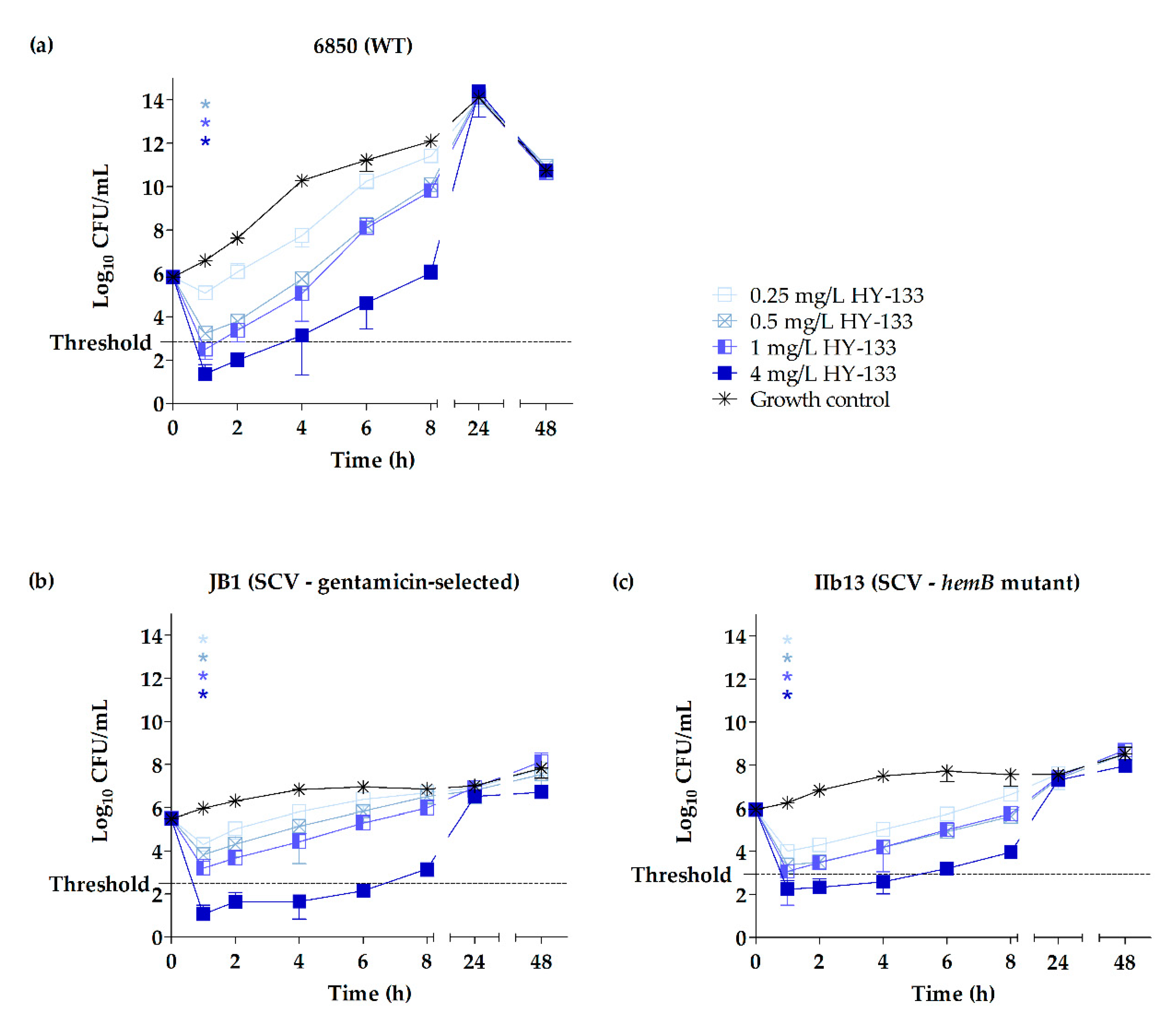
2.3. Time-Kill Studies
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Maintenance
4.2. Genotyping by PFGE
4.3. Antimicrobial Susceptibility Testing
4.4. Time-Kill Studies
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | Cell wall-binding domain |
| CHAP | Histidine-dependent amidohydrolases/peptidases |
| EAD | Enzymatically active domain |
| MBC50/90 | Minimum bactericidal concentration required to kill 50% and 90%, respectively, of the tested organisms |
| MIC50/90 | Minimum inhibitory concentration required to inhibit growth of 50% and 90%, respectively, of the tested organisms |
| SCV | Small-colony variant |
| TMP-SXT | Trimethoprim-sulfamethoxazole |
| WTA | Wall teichoic acid |
| WT | Wild type |
References
- Köck, R.; Becker, K.; Cookson, B.; van Gemert-Pijnen, J.E.; Harbarth, S.; Kluytmans, J.; Mielke, M.; Peters, G.; Skov, R.L.; Struelens, M.J.; et al. Methicillin-resistant Staphylococcus aureus (MRSA): Burden of disease and control challenges in Europe. Euro Surveill. 2010, 15, 19688. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Kaspar, U.; Kriegeskorte, A.; Schubert, T.; Peters, G.; Rudack, C.; Pieper, D.H.; Wos-Oxley, M.; Becker, K. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ. Microbiol. 2016, 18, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Von Eiff, C.; Becker, K.; Machka, K.; Stammer, H.; Peters, G. Nasal Carriage as a Source of Staphylococcus aureus Bacteremia. N. Engl. J. Med. 2001, 344, 11–16. [Google Scholar] [CrossRef]
- Wertheim, H.F.; Vos, M.C.; Ott, A.; van Belkum, A.; Voss, A.; Kluytmans, J.A.; van Keulen, P.H.; Vandenbroucke-Grauls, C.M.; Meester, M.H.; Verbrugh, H.A. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004, 364, 703–705. [Google Scholar] [CrossRef]
- Becker, K.; Schaumburg, F.; Fegeler, C.; Friedrich, A.W.; Köck, R.; Prevalence of Multiresistant Microorganisms (PMM) Study Group. Staphylococcus aureus from the German general population is highly diverse. Int. J. Med. Microbiol. 2017, 307, 21–27. [Google Scholar] [CrossRef]
- Köck, R.; Werner, P.; Friedrich, A.W.; Fegeler, C.; Becker, K.; Bindewald, O.; for the Prevalence of Multiresistant Microorganisms (PMM) Study Group. Persistence of nasal colonization with human pathogenic bacteria and associated antimicrobial resistance in the German general population. New Microbes New Infect. 2016, 9, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, H.; Becker, K.; Dohmen, P.M.; Petrosillo, N.; Spencer, M.; van Rijen, M.; Wechsler-Fördös, A.; Pujol, M.; Dubouix, A.; Garau, J. Staphylococcus aureus and surgical site infections: Benefits of screening and decolonization before surgery. J. Hosp. Infect. 2016, 94, 295–304. [Google Scholar] [CrossRef]
- Köck, R.; Becker, K.; Cookson, B.; van Gemert-Pijnen, J.E.; Harbarth, S.; Kluytmans, J.; Mielke, M.; Peters, G.; Skov, R.L.; Struelens, M.J.; et al. Systematic literature analysis and review of targeted preventive measures to limit healthcare-associated infections by meticillin-resistant Staphylococcus aureus. Euro Surveill. 2014, 19, 20860. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin. MMWR. Morb. Mortal. Wkly. Rep. 2002, 51, 565–567. [Google Scholar]
- Tsiodras, S.; Gold, H.S.; Sakoulas, G.; Eliopoulos, G.M.; Wennersten, C.; Venkataraman, L.; Moellering, R.C.; Ferraro, M.J. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 2001, 358, 207–208. [Google Scholar] [CrossRef]
- Mangili, A.; Bica, I.; Snydman, D.R.; Hamer, D.H. Daptomycin-Resistant, Methicillin-Resistant Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2005, 40, 1058–1060. [Google Scholar] [CrossRef]
- Schaumburg, F.; Peters, G.; Alabi, A.; Becker, K.; Idelevich, E.A. Missense mutations of PBP2a are associated with reduced susceptibility to ceftaroline and ceftobiprole in African MRSA. J. Antimicrob. Chemother. 2016, 71, 41–44. [Google Scholar] [CrossRef]
- Thomas, C.M.; Hothersall, J.; Willis, C.L.; Simpson, T.J. Resistance to and synthesis of the antibiotic mupirocin. Nat. Rev. Microbiol. 2010, 8, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Reagan, D.R.; Doebbeling, B.N.; Pfaller, M.A.; Sheetz, C.T.; Houston, A.K.; Hollis, R.J.; Wenzel, R.P. Elimination of Coincident Staphylococcus aureus Nasal and Hand Carriage with Intranasal Application of Mupirocin Calcium Ointment. Ann. Intern. Med. 1991, 114, 101–106. [Google Scholar] [CrossRef] [PubMed]
- White, D.G.; Collins, P.O.; Rowsell, R.B. Topical antibiotics in the treatment of superficial skin infections in general practice—A comparison of mupirocin with sodium fusidate. J. Infect. 1989, 18, 221–229. [Google Scholar] [CrossRef]
- Hill, R.L.; Duckworth, G.J.; Casewell, M.W. Elimination of nasal carriage of methicillin-resistant Staphylococcus aureus with mupirocin during a hospital outbreak. J. Antimicrob. Chemother. 1988, 22, 377–384. [Google Scholar] [CrossRef]
- Cederna, J.E.; Terpenning, M.S.; Ensberg, M.; Bradley, S.F.; Kauffman, C.A. Staphylococcus aureus Nasal Colonization in a Nursing Home: Eradication With Mupirocin. Infect. Control Hosp. Epidemiol. 1990, 11, 13–16. [Google Scholar] [CrossRef]
- Doebbeling, B.N.; Reagan, D.R.; Pfaller, M.A.; Houston, A.K.; Hollis, R.J.; Wenzel, R.P. Long-term Efficacy of Intranasal Mupirocin Ointment—A Prospective Cohort Study of Staphylococcus aureus Carriage. Arch. Intern. Med. 1994, 154, 1505–1508. [Google Scholar] [CrossRef]
- Yun, H.-J.; Lee, S.W.; Yoon, G.M.; Kim, S.Y.; Choi, S.; Lee, Y.S.; Choi, E.-C.; Kim, S.Y. Prevalence and mechanisms of low- and high-level mupirocin resistance in staphylococci isolated from a Korean hospital. J. Antimicrob. Chemother. 2003, 51, 619–623. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.B.; Gorwitz, R.J.; Jernigan, J.A. Mupirocin Resistance. Clin. Infect. Dis. 2009, 49, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Hetem, D.J.; Bonten, M.J.M. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J. Hosp. Infect. 2013, 85, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Coates, T.; Bax, R.; Coates, A. Nasal decolonization of Staphylococcus aureus with mupirocin: Strengths, weaknesses and future prospects. J. Antimicrob. Chemother. 2009, 64, 9–15. [Google Scholar] [CrossRef]
- Kavi, J.; Andrews, J.M.; Wise, R.; Smith, M.D.; Sanghrajka, M.; Lock, S. Mupirocin-Resistant Staphylococcus aureus. Lancet 1987, 330, 1472–1473. [Google Scholar] [CrossRef]
- Miller, M.H.; Wexler, M.A.; Steigbigel, N.H. Single and Combination Antibiotic Therapy of Staphylococcus aureus Experimental Endocarditis: Emergence of Gentamicin-Resistant Mutants. Antimicrob. Agents Chemother. 1978, 14, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Kriegeskorte, A.; Lorè, N.I.; Bragonzi, A.; Riva, C.; Kelkenberg, M.; Becker, K.; Proctor, R.A.; Peters, G.; Kahl, B.C. Thymidine-Dependent Staphylococcus aureus Small-Colony Variants Are Induced by Trimethoprim-Sulfamethoxazole (SXT) and Have Increased Fitness during SXT Challenge. Antimicrob. Agents Chemother. 2015, 59, 7265–7272. [Google Scholar] [CrossRef]
- Proctor, R.A.; von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.C.; Becker, K.; Löffler, B. Clinical Significance and Pathogenesis of Staphylococcal Small Colony Variants in Persistent Infections. Clin. Microbiol. Rev. 2016, 29, 401–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, R.A.; van Langevelde, P.; Kristjansson, M.; Maslow, J.N.; Arbeit, R.D. Persistent and Relapsing Infections Associated with Small-Colony Variants of Staphylococcus aureus. Clin. Infect. Dis. 1995, 20, 95–102. [Google Scholar] [CrossRef]
- Von Eiff, C.; Lubritz, G.; Heese, C.; Peters, G.; Becker, K. Effect of trimethoprim-sulfamethoxazole prophylaxis in AIDS patients on the formation of the small colony variant phenotype of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2004, 48, 191–194. [Google Scholar] [CrossRef]
- Sachse, F.; Becker, K.; von Eiff, C.; Metze, D.; Rudack, C. Staphylococcus aureus invades the epithelium in nasal polyposis and induces IL-6 in nasal epithelial cells in vitro. Allergy 2010, 65, 1430–1437. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Barcia-Macay, M.; Lemaire, S.; Tulkens, P.M. Cellular pharmacodynamics and pharmacokinetics of antibiotics: Current views and perspectives. Curr. Opin. Drug Discov. Dev. 2006, 9, 218–230. [Google Scholar]
- Hobby, G.L.; Dawson, M.H. Effect of Rate of Growth of Bacteria on Action of Penicillin. Proc. Soc. Exp. Biol. Med. 1944, 56, 181–184. [Google Scholar] [CrossRef]
- Kirby, W.M. Bacteriostatic And Lytic Actions Of Penicillin On Sensitive And Resistant Staphylococci. J. Clin. Invest. 1945, 24, 165–169. [Google Scholar] [CrossRef]
- Tuomanen, E.; Cozens, R.; Tosch, W.; Zak, O.; Tomasz, A. The Rate of Killing of Escherichia coli by β-Lactam Antibiotics Is Strictly Proportional to the Rate of Bacterial Growth. J. Gen. Microbiol. 1986, 132, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.; Lemaire, S.; van Bambeke, F.; Tulkens, P.M.; Hughes, D.; von Eiff, C.; Frimodt-Møller, N. Intra- and Extracellular Activities of Dicloxacillin and Linezolid against a Clinical Staphylococcus aureus Strain with a Small-Colony-Variant Phenotype in an In Vitro Model of THP-1 Macrophages and an In Vivo Mouse Peritonitis Model. Antimicrob. Agents Chemother. 2011, 55, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.; Herrmann, M.; Everding, A.S.; Koch, H.G.; Becker, K.; Harms, E.; Proctor, R.A.; Peters, G. Persistent Infection with Small Colony Variant Strains of Staphylococcus aureus in Patients with Cystic Fibrosis. J. Infect. Dis. 1998, 177, 1023–1029. [Google Scholar] [CrossRef]
- Von Eiff, C.; Bettin, D.; Proctor, R.A.; Rolauffs, B.; Lindner, N.; Winkelmann, W.; Peters, G. Recovery of Small Colony Variants of Staphylococcus aureus Following Gentamicin Bead Placement for Osteomyelitis. Clin. Infect. Dis. 1997, 25, 1250–1251. [Google Scholar] [CrossRef]
- Baumert, N.; von Eiff, C.; Schaaff, F.; Peters, G.; Proctor, R.A.; Sahl, H.-G. Physiology and Antibiotic Susceptibility of Staphylococcus aureus Small Colony Variants. Microb. Drug Resist. 2002, 8, 253–260. [Google Scholar] [CrossRef]
- Garcia, L.G.; Lemaire, S.; Kahl, B.C.; Becker, K.; Proctor, R.A.; Denis, O.; Tulkens, P.M.; van Bambeke, F. Antibiotic activity against small-colony variants of Staphylococcus aureus: Review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 2013, 68, 1455–1464. [Google Scholar] [CrossRef]
- Kriegeskorte, A.; König, S.; Sander, G.; Pirkl, A.; Mahabir, E.; Proctor, R.A.; von Eiff, C.; Peters, G.; Becker, K. Small colony variants of Staphylococcus aureus reveal distinct protein profiles. Proteomics 2011, 11, 2476–2490. [Google Scholar] [CrossRef]
- Kriegeskorte, A.; Grubmüller, S.; Huber, C.; Kahl, B.C.; von Eiff, C.; Proctor, R.A.; Peters, G.; Eisenreich, W.; Becker, K. Staphylococcus aureus small colony variants show common metabolic features in central metabolism irrespective of the underlying auxotrophism. Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A.; Kriegeskorte, A.; Kahl, B.C.; Becker, K.; Löffler, B.; Peters, G. Staphylococcus aureus Small Colony Variants (SCVs): A road map for the metabolic pathways involved in persistent infections. Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef]
- Bulger, R.J.; Bulger, R.E. Ultrastructure of Small Colony Variants of a Methicillin-Resistant Staphylococcus aureus. J. Bacteriol. 1967, 94, 1244–1246. [Google Scholar]
- Kahl, B.C.; Belling, G.; Reichelt, R.; Herrmann, M.; Proctor, R.A.; Peters, G. Thymidine-Dependent Small-Colony Variants of Staphylococcus aureus Exhibit Gross Morphological and Ultrastructural Changes Consistent with Impaired Cell Separation. J. Clin. Microbiol. 2003, 41, 410–413. [Google Scholar] [CrossRef]
- Forbes, S.; Latimer, J.; Bazaid, A.; McBain, A.J. Altered Competitive Fitness, Antimicrobial Susceptibility, and Cellular Morphology in a Triclosan-Induced Small-Colony Variant of Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 4809–4816. [Google Scholar] [CrossRef]
- Fenton, M.; McAuliffe, O.; O’Mahony, J.; Coffey, A. Recombinant bacteriophage lysins as antibacterials. Bioeng. Bugs 2010, 1, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashani, H.H.; Schmelcher, M.; Sabzalipoor, H.; Hosseini, E.S.; Moniri, R. Recombinant Endolysins as Potential Therapeutics against Antibiotic-Resistant Staphylococcus aureus: Current Status of Research and Novel Delivery Strategies. Clin. Microbiol. Rev. 2018, 31, e00071-17. [Google Scholar] [CrossRef]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2018, 53, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Lavigne, R. Breaking barriers: Expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol. 2015, 10, 377–390. [Google Scholar] [CrossRef]
- Gilmer, D.B.; Schmitz, J.E.; Thandar, M.; Euler, C.W.; Fischetti, V.A. The Phage Lysin PlySs2 Decolonizes Streptococcus suis from Murine Intranasal Mucosa. PLoS ONE 2017, 12, e0169180. [Google Scholar] [CrossRef] [PubMed]
- Lood, R.; Raz, A.; Molina, H.; Euler, C.W.; Fischetti, V.A. A Highly Active and Negatively Charged Streptococcus pyogenes Lysin with a Rare D-Alanyl-L-Alanine Endopeptidase Activity Protects Mice Against Streptococcal Bacteremia. Antimicrob. Agents Chemother. 2014, 58, 3073–3084. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Loomis, L.; Fischetti, V.A. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 2001, 98, 4107–4112. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, J.M.; Nelson, D.; Fischetti, V.A. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 2001, 294, 2170–2173. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Yu, J.; Huang, Y.; Zhang, X.-E.; Wei, H. Novel Chimeric Lysin with High-Level Antimicrobial Activity against Methicillin-Resistant Staphylococcus aureus In Vitro and In Vivo. Antimicrob. Agents Chemother. 2014, 58, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Totté, J.E.E.; van Doorn, M.B.; Pasmans, S.G.M.A. Successful Treatment of Chronic Staphylococcus aureus-Related Dermatoses with the Topical Endolysin Staphefekt SA.100: A Report of 3 Cases. Case Rep. Dermatol. 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Fan, J.; Zeng, Z.; Mai, K.; Yang, Y.; Feng, J.; Bai, Y.; Sun, B.; Xie, Q.; Tong, Y.; Ma, J. Preliminary treatment of bovine mastitis caused by Staphylococcus aureus, with trx-SA1, recombinant endolysin of S. aureus bacteriophage IME-SA1. Vet. Microbiol. 2016, 191, 65–71. [Google Scholar] [CrossRef]
- Idelevich, E.A.; von Eiff, C.; Friedrich, A.W.; Iannelli, D.; Xia, G.; Peters, G.; Peschel, A.; Wanninger, I.; Becker, K. In Vitro Activity against Staphylococcus aureus of a Novel Antimicrobial Agent, PRF-119, a Recombinant Chimeric Bacteriophage Endolysin. Antimicrob. Agents Chemother. 2011, 55, 4416–4419. [Google Scholar] [CrossRef]
- Idelevich, E.A.; Schaumburg, F.; Knaack, D.; Scherzinger, A.S.; Mutter, W.; Peters, G.; Peschel, A.; Becker, K. The Recombinant Bacteriophage Endolysin HY-133 Exhibits In Vitro Activity against Different African Clonal Lineages of the Staphylococcus aureus Complex, Including Staphylococcus schweitzeri. Antimicrob. Agents Chemother. 2016, 60, 2551–2553. [Google Scholar] [CrossRef]
- Knaack, D.; Idelevich, E.A.; Schleimer, N.; Molinaro, S.; Kriegeskorte, A.; Peters, G.; Becker, K. Bactericidal activity of bacteriophage endolysin HY-133 against Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, U.; de Haro Sautto, J.A.; Molinaro, S.; Peters, G.; Idelevich, E.A.; Becker, K. The Novel Phage-Derived Antimicrobial Agent HY-133 Is Active against Livestock-Associated Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2018. [Google Scholar] [CrossRef]
- Sharma, U.; Vipra, A.; Channabasappa, S. Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discov. Today 2018, 23, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shi, Y.; Ji, W.; Meng, X.; Zhang, J.; Wang, H.; Lu, C.; Sun, J.; Yan, Y. Application of a Bacteriophage Lysin To Disrupt Biofilms Formed by the Animal Pathogen Streptococcus suis. Appl. Environ. Microbiol. 2011, 77, 8272–8279. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-S.; Lee, S.-J.; Jun, S.Y.; Yoon, S.J.; Kang, S.H.; Paik, H.R.; Kang, J.O.; Choi, Y.-J. Antibacterial and biofilm removal activity of a podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Appl. Microbiol. Biotechnol. 2010, 86, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Ross, R.P.; Meaney, W.; Fitzgerald, G.F.; Elbreki, M.F.; Coffey, A. Potential of the Polyvalent Anti-Staphylococcus Bacteriophage K for Control of Antibiotic-Resistant Staphylococci from Hospitals. Appl. Environ. Microbiol. 2005, 71, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Dong, S.; Baker, J.R.; Foster-Frey, J.; Pritchard, D.G.; Donovan, D.M. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol. Lett. 2009, 294, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting Chromosomal DNA Restriction Patterns Produced by Pulsed-Field Gel Electrophoresis: Criteria for Bacterial Strain Typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar]
- CLSI Supplement M100. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; ISBN 1562388045.
- Abu-Qatouseh, L.; Chinni, S.; Seggewiß, J.; Proctor, R.A.; Brosius, J.; Rozhdestvensky, T.S.; Peters, G.; von Eiff, C.; Becker, K. Identification of differentially expressed small non-protein-coding RNAs in Staphylococcus aureus displaying both the normal and the small-colony variant phenotype. J. Mol. Med. 2010, 88, 565–575. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Kokai-Kun, J.F.; Kristian, S.A.; Chanturiya, T.; Kalbacher, H.; Gross, M.; Nicholson, G.; Neumeister, B.; Mond, J.J.; Peschel, A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 2004, 10, 243–245. [Google Scholar] [CrossRef]
- Piechowicz, L.; Garbacz, K.; Galiński, J. Staphylococcus aureus of phage type 187 isolated from people occurred to be a genes carrier of enterotoxin C and toxic shock syndrome toxin-1 (TSST-1). Int. J. Hyg. Environ. Health 2008, 211, 273–282. [Google Scholar] [CrossRef]
- Von Eiff, C.; Becker, K.; Metze, D.; Lubritz, G.; Hockmann, J.; Schwarz, T.; Peters, G. Intracellular Persistence of Staphylococcus aureus Small-Colony Variants within Keratinocytes: A Cause for Antibiotic Treatment Failure in a Patient with Darier’s Disease. Clin. Infect. Dis. 2001, 32, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Von Eiff, C.; McNamara, P.; Becker, K.; Bates, D.; Lei, X.-H.; Ziman, M.; Bochner, B.R.; Peters, G.; Proctor, R.A. Phenotype Microarray Profiling of Staphylococcus aureus menD and hemB Mutants with the Small-Colony-Variant Phenotype. J. Bacteriol. 2006, 188, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.D.R.; Brandão, A.; Akturk, E.; Santos, S.B.; Azeredo, J. Characterization of a New Staphylococcus aureus Kayvirus Harboring a Lysin Active against Biofilms. Viruses 2018, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- NCCLS M26-A. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; The National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999; Volume 19, ISBN 1562383841.
- Garcia, L.G.; Lemaire, S.; Kahl, B.C.; Becker, K.; Proctor, R.A.; Tulkens, P.M.; Van Bambeke, F. Intracellular forms of menadione-dependent small-colony variants of methicillin-resistant Staphylococcus aureus are hypersusceptible to beta-lactams in a THP-1 cell model due to cooperation between vacuolar acidic pH and oxidant species. J. Antimicrob. Chemother. 2012, 67, 2873–2881. [Google Scholar] [CrossRef]
- Walsh, C. Antibiotics: Actions, Origins, Resistance; American Society for Microbiology (ASM): Washington, WA, USA, 2003; ISBN 1555812546. [Google Scholar]
- Von Eiff, C.; Heilmann, C.; Proctor, R.; Woltz, C.; Peters, G.; Götz, F. A Site-Directed Staphylococcus aureus hemB Mutant Is a Small-Colony Variant Which Persists Intracellularly. J. Bacteriol. 1997, 179, 4706–4712. [Google Scholar] [CrossRef] [PubMed]
- DeHart, H.P.; Heath, H.E.; Heath, L.S.; LeBlanc, P.A.; Sloan, G.L. The Lysostaphin Endopeptidase Resistance Gene (epr) Specifies Modification of Peptidoglycan Cross Bridges in Staphylococcus simulans and Staphylococcus aureus. Appl. Environ. Microbiol. 1995, 61, 1475–1479. [Google Scholar]
- Gründling, A.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus Mutants with Increased Lysostaphin Resistance. J. Bacteriol. 2006, 188, 6286–6297. [Google Scholar] [CrossRef]
- Schuch, R.; Nelson, D.; Fischetti, V.A. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 2002, 418, 884–889. [Google Scholar] [CrossRef]
- Pastagia, M.; Euler, C.; Chahales, P.; Fuentes-Duculan, J.; Krueger, J.G.; Fischetti, V.A. A Novel Chimeric Lysin Shows Superiority to Mupirocin for Skin Decolonization of Methicillin-Resistant and -Sensitive Staphylococcus aureus Strains. Antimicrob. Agents Chemother. 2011, 55, 738–744. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [Green Version]
- Fischetti, V.A. Bacteriophage lytic enzymes: Novel anti-infectives. Trends Microbiol. 2005, 13, 491–496. [Google Scholar] [CrossRef]
- Shen, Y.; Barros, M.; Vennemann, T.; Gallagher, D.T.; Yin, Y.; Linden, S.B.; Heselpoth, R.D.; Spencer, D.J.; Donovan, D.M.; Moult, J.; et al. A bacteriophage endolysin that eliminates intracellular streptococci. Elife 2016, 5, e13152. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Medina, E.; Hussain, M.; Völker, W.; Heitmann, V.; Niemann, S.; Holzinger, D.; Roth, J.; Proctor, R.A.; Becker, K.; et al. Staphylococcus aureus phenotype switching: An effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 2011, 3, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Balwit, J.M.; Langevelde, P.v.; Vann, J.M.; Proctor, R.A. Gentamicin-Resistant Menadione and Hemin Auxotrophic Staphylococcus aureus Persist within Cultured Endothelial Cells. J. Infect. Dis. 1994, 170, 1033–1037. [Google Scholar] [CrossRef]
- Moenninghoff, C. Untersuchungen zur klinischen Relevanz von Staphylococcus aureus Small Colony Variants bei Patienten mit Osteomyelitis; Westfälische Wilhelms-Universität: Münster, Germany, 2006. [Google Scholar]
- Becker, K.; Kahl, B.; von Eiff, C.; Roth, R.; Peters, G. Exotoxin production by small colony variants (SCV) of Staphylococcus aureus. In Proceedings of the 9th European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany, 16–19 May 1999; pp. 333–334. [Google Scholar]
- Kriegeskorte, A.; Ballhausen, B.; Idelevich, E.A.; Köck, R.; Friedrich, A.W.; Karch, H.; Peters, G.; Becker, K. Human MRSA Isolates with Novel Genetic Homolog, Germany. Emerg. Infect. Dis. 2012, 18, 1016–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipp, F.; Becker, K.; Peters, G.; von Eiff, C. Evaluation of Different Methods To Detect Methicillin Resistance in Small-Colony Variants of Staphylococcus aureus. J. Clin. Microbiol. 2004, 42, 1277–1279. [Google Scholar] [CrossRef]
- Becker, K.; van Alen, S.; Idelevich, E.A.; Schleimer, N.; Seggewiß, J.; Mellmann, A.; Kaspar, U.; Peters, G. Plasmid-Encoded Transferable mecB-Mediated Methicillin Resistance in Staphylococcus aureus. Emerg. Infect. Dis. 2018, 24, 242–248. [Google Scholar] [CrossRef]
- Vann, J.M.; Proctor, R.A. Ingestion of Staphylococcus aureus by Bovine Endothelial Cells Results in Time- and Inoculum-Dependent Damage to Endothelial Cell Monolayers. Infect. Immun. 1987, 55, 2155–2163. [Google Scholar]
- Vaudaux, P.; Francois, P.; Bisognano, C.; Kelley, W.L.; Lew, D.P.; Schrenzel, J.; Proctor, R.A.; McNamara, P.J.; Peters, G.; von Eiff, C. Increased Expression of Clumping Factor and Fibronectin-Binding Proteins by hemB Mutants of Staphylococcus aureus Expressing Small Colony Variant Phenotypes. Infect. Immun. 2002, 70, 5428–5437. [Google Scholar] [CrossRef]
- Goering, R.V.; Winters, M.A. Rapid Method for Epidemiological Evaluation of Gram-Positive Cocci by Field Inversion Gel Electrophoresis. J. Clin. Microbiol. 1992, 30, 577–580. [Google Scholar] [PubMed]
- CLSI M07. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 1562388363. [Google Scholar]

| Antimicrobial Agent | Growth Phase | Phenotype (No. of Strains) | Median MIC (mg/L) 1 | Median MBC (mg/L) 1 | ||||
|---|---|---|---|---|---|---|---|---|
| 50% | 90% | Range | 50% | 90% | Range | |||
| HY-133 | Stationary growth 2 | WT (12) | 0.12 | 0.5 | 0.12–0.5 | 0.12 | 0.5 | 0.12–0.5 |
| SCV (12) | 0.25 | 0.5 | 0.12–0.5 | 0.25 | 0.5 | 0.12–0.5 | ||
| Logarithmic growth 3 | WT (12) | 0.25 | 0.5 | 0.25–0.5 | 0.25 | 0.5 | 0.25–0.5 | |
| SCV (12) | 0.12 | 0.5 | 0.12–0.5 | 0.12 | 0.5 | 0.12–0.5 | ||
| Oxacillin | Stationary growth 2 | WT (12) | 0.5 | 1 | 0.25–2 | 0.5 | 1 | 0.25–2 |
| SCV (12) | 0.25 | 1 | 0.25–1 | 0.25 | 1 | 0.25–2 | ||
| Logarithmic growth 3 | WT (12) | 0.5 | 1 | 0.25–1 | 0.5 | 1 | 0.25–2 | |
| SCV (12) | 0.25 | 0.5 | 0.12–1 | 0.25 | 1 | 0.25–1 | ||
| Antimicrobial Agent | Growth Phase | Phenotype | Median MIC (mg/L) | Median MBC (mg/L) |
|---|---|---|---|---|
| HY-133 | Stationary growth 1 | 6850 (WT) | 0.12 | 0.12 |
| JB1 (selected SCV) | 0.25 | 0.25 | ||
| IIb13 (mutant SCV) | 0.12 | 0.12 | ||
| Logarithmic growth 2 | 6850 (WT) | 0.25 | 0.25 | |
| JB1 (selected SCV) | 1 | 1 | ||
| IIb13 (mutant SCV) | 0.25 | 0.25 | ||
| Oxacillin | Stationary growth 1 | 6850 (WT) | 0.5 | 0.5 |
| JB1 (selected SCV) | 0.06 | 0.06 | ||
| IIb13 (mutant SCV) | 0.06 | 0.06 | ||
| Logarithmic growth 2 | 6850 (WT) | 0.5 | 0.5 | |
| JB1 (selected SCV) | 0.03 | 0.03 | ||
| IIb13 (mutant SCV) | 0.03 | 0.06 |
| Strain (Phenotype) | Growth Reduction | Time (h) when Respective Growth Reduction Was Reached for the Following Concentrations (mg/L) of Antimicrobial Used | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HY-133 | Oxacillin | ||||||||
| 0.25 | 0.5 | 1 | 4 | 0.25 | 0.5 | 1 | 4 | ||
| OM299-1 (WT) | 90% | NR | NR | 1 | 1 | 8 | 4 | 2 | 2 |
| 99% | NR | NR | 1 | 1 | NR | 4 | 4 | 4 | |
| 99.9% | NR | NR | NR | 1 | NR | 8 | 8 | 8 | |
| OM299-2 (SCV) | 90% | NR | 1 | 1 | 1 | 6 | 6 | 4 | 4 |
| 99% | NR | NR | 1 | 1 | 24 | 8 | 8 | 8 | |
| 99.9% | NR | NR | 1 | 1 | 24 | 24 | 24 | 24 | |
| 4652I (WT) | 90% | NR | 1 | 1 | 1 | NR | 4 | 4 | 2 |
| 99% | NR | NR | 1 | 1 | NR | 8 | 4 | 4 | |
| 99.9% | NR | NR | 1 | 1 | NR | 24 | 8 | 8 | |
| 4652II (SCV) | 90% | 1 | 1 | 1 | 1 | 4 | 4 | 4 | 2 |
| 99% | 1 | 1 | 1 | 1 | 8 | 4 | 4 | 4 | |
| 99.9% | NR | NR | 1 | 1 | NR | 6 | 6 | 4 | |
| 6850 (WT) | 90% | NR | 1 | 1 | 1 | NP | NP | NP | NP |
| 99% | NR | 1 | 1 | 1 | NP | NP | NP | NP | |
| 99.9% | NR | NR | 1 | 1 | NP | NP | NP | NP | |
| JB1 (SCV) | 90% | 1 | 1 | 1 | 1 | NP | NP | NP | NP |
| 99% | NR | NR | 1 | 1 | NP | NP | NP | NP | |
| 99.9% | NR | NR | NR | 1 | NP | NP | NP | NP | |
| IIb13 (SCV) | 90% | 1 | 1 | 1 | 1 | NP | NP | NP | NP |
| 99% | NR | 1 | 1 | 1 | NP | NP | NP | NP | |
| 99.9% | NR | NR | NR | 1 | NP | NP | NP | NP | |
| Strain No. | Phenotype | Underlying Disease/Description | Source | Reference |
|---|---|---|---|---|
| A22616/5 | WT | Osteomyelitis | Tissue 1 | [39] |
| A22616/3 | SCV | Tissue 1 | [39] | |
| OM1a | WT | Sternoclavicular joint arthritis with abscess | Tissue 1 | [39] |
| OM1b | SCV | Tissue 1 | [39] | |
| OM184/1 | WT | Acute osteomyelitis | Bone (distal radius) | [89] |
| OM184/2 | SCV | Bone (distal radius) | [89] | |
| OM299-1 2 | WT | Femur osteomyelitis | Tissue (femur) | [39] |
| OM299-2 2 | SCV | Tissue (femur) | [39] | |
| OM420/1 | WT | Knee arthrodesis-associated chronic osteomyelitis | Tissue (tibia) | [89] |
| OM420/3 | SCV | Tissue (tibia) | [89] | |
| 4652I 2 | WT | Acute osteomyelitis with tibia abscess | Abscess (tibia) | [89] |
| 4652II 2 | SCV | Abscess (tibia) | [89] | |
| K3515I | WT | Sepsis | Blood | This study |
| K3515II | SCV | Blood | This study | |
| A9380II | WT | Lumbar spondylitis | Swab (lumbar disc) | This study |
| A9379I | SCV | Swab (lumbar disc) | [89] | |
| OM372/1 | WT | Chronic osteomyelitis | Tissue (femur) | This study |
| OM372/2 | SCV | Tissue (femur) | [89] | |
| 14799 | WT | Chronic osteomyelitis | Tissue (femur exostosis) | This study |
| OM40/1 | SCV | Tissue (femur exostosis) | [89] | |
| OM234 | WT | Hip osteoarthritis | Swab (joint) | This study |
| OM235/2 | SCV | Swab (bone) | This study | |
| A5382I | WT | Hip TEP infection | Swab (joint) | This study |
| A5382III | SCV | Swab (joint) | This study | |
| 6850 2 | WT | Skin abscess | - | [94] |
| JB1 2 | SCV | SCV, in vitro selected with gentamicin from 6850 | - | [88] |
| IIb13 2 | SCV | ΔhemB (hemB::ermB) mutant from 6850 | - | [95] |
| ATCC 29213 | WT | Reference strain, S. aureus subsp. aureus | Wound | ATCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schleimer, N.; Kaspar, U.; Knaack, D.; von Eiff, C.; Molinaro, S.; Grallert, H.; Idelevich, E.A.; Becker, K. In Vitro Activity of the Bacteriophage Endolysin HY-133 against Staphylococcus aureus Small-Colony Variants and Their Corresponding Wild Types. Int. J. Mol. Sci. 2019, 20, 716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030716
Schleimer N, Kaspar U, Knaack D, von Eiff C, Molinaro S, Grallert H, Idelevich EA, Becker K. In Vitro Activity of the Bacteriophage Endolysin HY-133 against Staphylococcus aureus Small-Colony Variants and Their Corresponding Wild Types. International Journal of Molecular Sciences. 2019; 20(3):716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030716
Chicago/Turabian StyleSchleimer, Nina, Ursula Kaspar, Dennis Knaack, Christof von Eiff, Sonja Molinaro, Holger Grallert, Evgeny A. Idelevich, and Karsten Becker. 2019. "In Vitro Activity of the Bacteriophage Endolysin HY-133 against Staphylococcus aureus Small-Colony Variants and Their Corresponding Wild Types" International Journal of Molecular Sciences 20, no. 3: 716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030716






