Abscisic Acid Regulates the 3-Hydroxy-3-methylglutaryl CoA Reductase Gene Promoter and Ginsenoside Production in Panax quinquefolium Hairy Root Cultures
Abstract
:1. Introduction
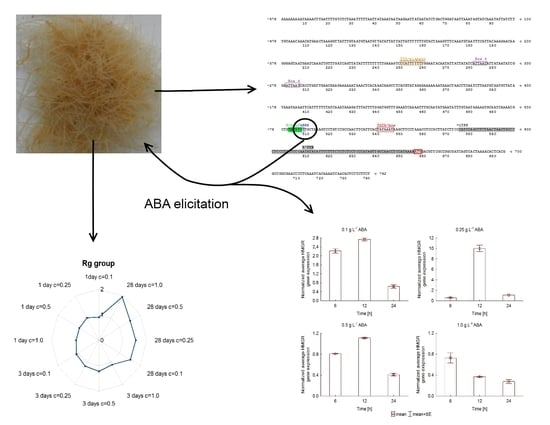
2. Results
2.1. In silico Analysis of P. quinquefolium HMGR Promoter
2.2. The Effect of ABA Elicitation on Hairy Roots of P. quinquefolium Growth
2.3. The Effect of ABA Elicitation on Ginsenoside Production in Hairy Roots of P. quinquefolium
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Measurement of Hairy Root Culture Growth
4.3. Isolation of Genomic DNA
4.4. Promoter Isolation
4.5. Cloning and Sequencing of the PqHMGR Gene Promoter
4.6. In Silico Analysis of the P. quinquefolium HMGR Promoter
4.7. RNA Isolation and Reverse Transcriptase (RT)-PCR Analysis
4.8. Qualitative Analysis
4.9. Quantitative Analysis by Real-Time PCR
4.10. Abscisic acid Treatment
4.11. HPLC Analysis of Ginsenosides
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Rhee, D.K. Effects of ginseng on stress-related depression, anxiety and the hypothalamic-pituitary-adrenal axis. J. Ginseng Res. 2017, 41, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.A.; Son, D.; Park, D.; Jung, E. Anti-skin-aging activity of a standardized extract from Panax ginseng leaves in vitro and in human volunteer. Cosmetics 2017, 4, 18. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, E.J.; Cheon, J.M.; Nam, K.J.; Oh, T.H.; Kim, K.S. Antioxidant and hepatoprotective effects of fermented red ginseng against high fat diet-induced hyperlipidemia in rats. Lab. Anim. Res. 2018, 32, 217–223. [Google Scholar] [CrossRef]
- Yang, W.Z.; Hu, Y.; Wu, W.Y.; Ye, M.; Guo, D.A. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry 2014, 106, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Hu, Z.F.; Zhang, T.T.; Gu, A.D.; Gong, T.; Zhu, P. Progress on the studies of the key enzymes of ginsenoside biosynthesis. Molecules 2018, 23, 589. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Nuruzzaman, M.; Xiu, H.; Huang, J.; Wu, K.; Chen, X.; Li, J.; Wang, L.; Jeong, J.H.; Park, S.J.; et al. Transcriptome analysis of methyl asmonate-elicited Panax ginseng adventitious roots to discover putative ginsenoside biosynthesis and transport genes. Int. J. Mol. Sci. 2015, 16, 3035–3057. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, O.R.; Oh, J.Y.; Jang, M.G.; Yang, D.C. Functional analysis of 3-hydroxy-3-methylglutaryl coenzyme a reductase encoding genes in triterpene saponin-producing ginseng. Plant Physiol. 2014, 165, 373–387. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, C.; Chen, S. Identification and expression analysis of a 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from American ginseng. POJ 2012, 5, 414–420. [Google Scholar]
- Xu, J.; Chu, Y.; Liao, B.; Xiao, S.; Yin, Q.; Bai, R.; Su, H.; Dong, L.; Li, X.; Qian, J.; et al. Panax ginseng genome examination for ginsenoside biosynthesis. Gigascience 2017, 6, 1–15. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Wu, Q.; Luo, H.; Sun, Y.; Song, J.; Lui, E.M.K.; Chen, S. De novo sequencing and analysis of American ginseng root transcriptome using a GS FLX Titanium platform to discover putative genes involved in ginsenoside biosynthesis. BMC Genom. 2010, 11, 262. [Google Scholar] [CrossRef]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004, 5, e248. [Google Scholar] [CrossRef] [PubMed]
- Leng, P.; Yuan, B.; Guo, Y. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Langhansová, L.; Konrádová, H.; Vaněk, T. Polyethylene glycol and abscisic acid improve maturation and regeneration of Panax ginseng somatic embryos. Plant Cell Rep. 2004, 22, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulla, R.K.; Kim, Y.J.; Parvin, S.; Shim, J.S.; Lee, J.H.; Kim, Y.J.; In, J.G.; Senthil, K.S.; Yang, D.C. Isolation of S-adenosyl-l-methionine synthetase gene from Panax ginseng C.A. Meyer and analysis of its response to abiotic stresses. Physiol. Mol. Biol. Plants 2009, 15, 267–275. [Google Scholar] [CrossRef]
- Naruzzman, M.; Cao, H.; Xiu, H.; Luo, T.; Li, J.; Chen, X.; Luo, J.; Luo, Z. Transcriptomics-based identification of WRKY genes and characterization of a salt and hormone-responsive PgWRKY1 gene in Panax ginseng. Acta Biochim. Biophys. Sin. 2016, 48, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Xiu, H.; Naruzzman, M.; Guo, X.; Cao, H.; Huang, J.; Chen, X.; Wu, K.; Zhang, R.; Hung, Y.; Luo, J.; et al. Molecular cloning and expression analysis pf eight PgWRKY genes in Panax ginseng responsive to salt and hormones. Int. J. Mol. Sci. 2016, 17, 319. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, J.; Cao, H.Z.; Xie, X.L.; Huang, J.J.; Chen, X.H.; Luo, Z.Y. Isolation and characterization of LHT-type plant amino acid transporter gene from Panax ginseng Meyer. J. Ginseng Res. 2013, 37, 361–370. [Google Scholar] [CrossRef]
- Afrin, S.; Zhu, J.; Cao, H.; Huang, J.; Xiu, H.; Luo, T.; Luo, Z. Molecular cloning and expression profile of an abiotic stress and hormone responsive MYB transcription factor gene from Panax ginseng. Acta Biochim. Biophys. Sin. 2015, 47, 267–277. [Google Scholar] [CrossRef]
- Gupta, S.K.; Liu, R.B.; Liaw, S.Y.; Chan, H.S.; Tsay, H.S. Enhanced tanshinone production in hairy roots of ‘Salvia miltiorrhiza Bunge’ under the influence of plant growth regulators in liquid culture. Bot. Stud. 2011, 52, 435–443. [Google Scholar]
- Hao, G.; Ji, H.; Li, Y.; Shi, R.; Wang, J.; Feng, L.; Huang, L. Exogenous ABA and polyamines enhanced salvianolic acids contents in hairy root cultures of Salvia miltiorrhiza Bge. f. alba. POJ 2012, 5, 446–452. [Google Scholar]
- Shahmuradov, I.A.; Gammerman, A.J.; Hancock, J.M.; Bramley, P.M.; Solovyev, V.V. PlantProm: A database of plant promoter sequences. Nucleic Acids Res. 2003, 31, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Shahmuradov, I.A.; Solovyev, V.V.; Gammerman, A. Plant promoter prediction with confidence estimation. Nucleic Acids Res. 2005, 33, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [Green Version]
- Kristiansson, E.; Thorsen, M.; Tamas, M.J.; Nerman, O. Evolutionary forces act on promoter length: Identification of enriched cis-regulatory elements. Mol. Biol. Evol. 2009, 26, 1299–1307. [Google Scholar] [CrossRef]
- Cao, X.; Jacobsen, S.E. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proc. Natl. Acad. Sci. USA 2002, 99 (Suppl. 4), 16491–16498. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Harris, C.J.; Liu, Q.; Liu, W.; Ausin, I.; Long, Y.; Xiao, L.; Feng, L.; Chen, X.; Xie, Y.; et al. Large-scale comparative epigenomics reveals hierarchical regulation of non-CG methylation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E1069–E1074. [Google Scholar] [CrossRef]
- Ashikawa, I. Gene-associated CpG islands in plants as revealed by analyses of genomic sequences. Plant J. 2001, 26, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Lin, J.-Y.; Hur, J.; Pelletier, J.M.; Baden, R.; Pellegrini, M.; Harada, J.J.; Goldberg, R.B. Seed genome hypomethylated regions are enriched in transcription factor genes. Proc. Natl. Acad. Sci. USA 2018, 115, E8315–E8322. [Google Scholar] [CrossRef]
- Vinces, M.D.; Legendre, M.; Caldara, M.; Hagihara, M.; Verstrepen, K.J. Unstable tandem repeats in promoters confer transcriptional evolvability. Science 2009, 324, 1213–1216. [Google Scholar] [CrossRef]
- Jansen, A.; Gemayel, R.; Verstrepen, K.J. Unstable microsatellite repeats facilitate rapid evolution of coding and regulatory sequences. Genome Dyn. 2012, 7, 108–125. [Google Scholar]
- Workman, J.L. Nucleosome displacement in transcription. Genes Dev. 2006, 20, 2009–2017. [Google Scholar] [CrossRef] [Green Version]
- Bolton, K.A.; Ross, J.P.; Grice, D.M.; Bowden, N.A.; Holliday, E.G.; Avery-Kiejda, K.A.; Scott, R.J. STaRRRT: A table of short tandem repeats in regulatory regions of the human genome. BMC Genom. 2013, 14, 795. [Google Scholar] [CrossRef]
- Kaur, G.; Pati, P.K. Analysis of cis-acting regulatory elements of Respiratory burst oxidase homolog (Rboh) gene families in Arabidopsis and rice provides clues for their diverse functions. Comput. Biol. Chem. 2016, 62, 104–118. [Google Scholar] [CrossRef]
- Daraselia, N.D.; Tarchevskaya, S.; Narita, J.O. The promoter for tomato 3-hydroxy-3-methylglutaryl coenzyme a reductase gene 2 has unusual regulatory elements that direct high-level expression. Plant Physiol. 1996, 112, 727–733. [Google Scholar] [CrossRef]
- Jain, A.K.; Vincent, R.M.; Nessler, C.L. Molecular characterization of a hydroxymethylglutaryl-CoA reductase gene from mulberry (Morus alba L.). Plant Mol. Biol. 2000, 42, 559–569. [Google Scholar] [CrossRef]
- Kawoosa, T.; Gahlan, P.; Devi, A.S.; Kumar, S. The GATA and SORLIP motifs in the 3-hydroxy-3-methylglutaryl-CoA reductase gene of Picrorhiza kurrooa for the control of light-mediated expression. Funct. Integr. Genom. 2014, 14, 191–203. [Google Scholar] [CrossRef]
- Liao, Y.; Xu, F.; Huang, X.; Zhang, W.; Cheng, H.; Li, L.; Cheng, S.; Shen, Y. Promoter analysis and transcriptional profiling of Gingko biloba 3-hydroxy-3-methylglutaryl coenzyme A reductase (GbHMGR) gene in abiotic stress responses. Not. Bot. Horti Agrobot. 2015, 43, 25–34. [Google Scholar]
- Seetha, K.; Banerjee, N.S.; Omkumar, R.V.; Purushothama, M.G. Cloning and characterization of partial promoter of HMGCoA reductase from Andrographis paniculata (Burm.f.) Wall.ex Nees—A tropical medicinal plant. J. Plant Biochem. Biotechnol. 2005, 14, 41–44. [Google Scholar] [CrossRef]
- Szymczyk, P.; Grąbkowska, R.; Skała, E.; Żebrowska, M.; Balcerczak, E.; Jeleń, A. Isolation and characterization of a 3-hydroxy-3-methylglutaryl-coenzyme A reductase gene promoter from Salvia miltiorrhiza. J. Plant Biochem. Biotechnol. 2018, 27, 223–236. [Google Scholar]
- Ma, Y.; Cao, J.; He, J.; Chen, Q.; Li, X.; Yang, Y. Molecular mechanisms for the regulation of ABA homeostasis during plant development and stress responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar] [CrossRef]
- Cao, X.Y.; Li, C.-G.; Mao, Q.; Zheng, Z.J.; Jiang, J.H. Molecular cloning and expression analysis of a leaf-specific expressing 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase gene from Michelia chapensis Dandy. J. Med. Plant Res. 2011, 5, 3868–3875. [Google Scholar]
- Kang, M.K.; Park, K.S.; Choi, D. Coordinated expression in defense-related genes by TMV infection or salicylic acid treatment in tobacco. Mol. Cells 1998, 8, 388–392. [Google Scholar]
- Liao, P.; Zhou, W.; Zhang, L.; Wang, J.; Yan, X.; Zhang, Y.; Zhang, R.; Li, L.; Zhou, G.; Kai, G. Molecular cloning, characterization and expression analysis of a new gene encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase from Salvia miltiorrhiza. Acta Physiol. Plant. 2009, 31, 565–572. [Google Scholar] [CrossRef]
- Lv, D.M.; Zhang, T.T.; Deng, S.; Zhang, Y.H. Functional analysis of the Malus domestica MdHMGR2 gene promoter in transgenic Arabidopsis thaliana. Biol. Plant. 2016, 60, 667–676. [Google Scholar] [CrossRef]
- Xu, W.; Yu, Y.; Zhou, Q.; Ding, J.; Dai, L.; Xie, X.; Xu, Y.; Zhang, C.; Wang, Y. Expression pattern, genomic structure, and promoter analysis of the gene encoding stilbene synthase from Chinese wild Vitis pseudoreticulata. J. Exp. Bot. 2011, 62, 2745–2761. [Google Scholar] [CrossRef]
- Lin, P.C.; Hwang, S.G.; Endo, A.; Okamoto, M.; Koshiba, T.; Cheng, W.H. Ectopic Expression of abscisic acid 2/glucose insensitive 1 in Arabidopsis Promotes Seed Dormancy and Stress Tolerance. Plant Physiol. 2007, 143, 745–758. [Google Scholar] [CrossRef]
- Choi, H.; Hong, J.; Ha, J.; Kang, J.; Kim, S.Y. ABFs, a Family of ABA-responsive element binding factors. J. Biol. Chem. 2000, 275, 1723–1730. [Google Scholar] [CrossRef]
- Kumar, M.S.S.; Mawlong, I.; Ali, K.; Tyagi, A. Regulation of phytosterol biosynthetic pathway during drought stress in rice. Plant Physiol. Biochem. 2018, 129, 11–20. [Google Scholar] [CrossRef]
- Jing, F.; Zhang, L.; Li, M.; Tang, Y.; Wang, Y.; Wang, Y.; Wang, Q.; Pan, Q.; Wang, G.; Tang, K. Abscisic acid (ABA) treatment increases artemisinin content in Artemisia annua by enhancing the expression of genes in artemisinin biosynthesis pathway. Biologia 2009, 64, 319–323. [Google Scholar] [CrossRef]
- Yin, J.; Wang, L.; Huang, Y.; Mu, Y.; Lv, S. Authentication of Panax ginseng from different regions 2017. RSC Adv. 2017, 7, 55646–55652. [Google Scholar] [CrossRef]
- Luo, J.; Liu, L.; Wu, C.D. Enhancement of paclitaxel production by abscisic acid in cell suspension cultures of Taxus Chinensis. Biotechnol. Lett. 2001, 23, 1345–1348. [Google Scholar] [CrossRef]
- Sun, D.Y.; Yin, Z.J.; Wu, S.J.; Su, J.; Shi, S.; Wu, H.; Xiao, F.H.; Qi, J.L.; Liu, Z.; Pang, Y.J.; et al. Effects of abscisic acid on the secondary metabolism of cultured Onosma paniculatum cells. Russ. J. Plant Physiol. 2007, 4, 530–535. [Google Scholar] [CrossRef]
- Murcia, G.; Fontana, A.; Pontin, M.; Baraldi, R.; Bertazza, G.; Piccoli, P.N. ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine. Phytochemistry 2017, 135, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Jamalian, S.; Gholami, M.; Esna-Ashari, M. Abscisic acid-mediated leaf phenolic compounds, plant growth and yield is strawberry under different salt stress regimes. Theor. Exp. Plant Phys. 2013, 25, 291–299. [Google Scholar]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The biphasic root growth response to abscisic acid in Arabidopsis involves interaction with ethylene and auxin signalling pathways. Front Plant Sci. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Łuczkiewicz, M.; Kokotkiewicz, A. Genista tinctoria hairy root cultures for selective production of isoliquiritigenin. Z. Naturforsch. C 2005, 60, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G. Nitrogen and phosphorus as the factors affecting ginsenoside production in hairy root cultures of Panax quinquefolium cultivated in shake flasks and nutrient sprinkle bioreactor. Acta Physiol. Plant. 2016, 38, 149. [Google Scholar] [CrossRef]
- Khan, S.; Qureshi, M.I.; Kamaluddin Alam, T.; Abdin, M.Z. Protocol for isolation of genomic DNA from dry and fresh roots of medicinal plants suitable for RAPD and restriction digestion. Afr. J. Biotechnol. 2007, 6, 175–178. [Google Scholar]
- Siebert, P.D.; Chenchik, A.; Kellogg, D.E.; Lukyanov, K.A.; Lukyanov, S.A. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 1995, 23, 1087–1088. [Google Scholar] [CrossRef] [Green Version]
- Kibbe, W.A. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 2007, 35, W43–W46. [Google Scholar] [CrossRef] [PubMed]
- Birnboim, H.C.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.C.; Lee, T.Y.; Huang, H.D.; Huang, H.Y.; Pan, R.L. PlantPAN Plant Promoter analysis navigator, for identifying combinatorial cis-regulatory elements with distance constraint in plant gene group. BMC Genom. 2008, 9, 561–575. [Google Scholar] [CrossRef]
- Chow, C.N.; Zheng, H.Q.; Wu, N.Y.; Chien, C.H.; Huang, H.D.; Lee, T.Y.; Chiang-Hsieh, Y.-F.; Hou, P.-F.; Yang, T.Y.; Chang, W.C. PlantPAN 2.0: An update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res. 2016, 44, D1154–D1160. [Google Scholar] [CrossRef]
- Solovyev, V.V.; Shahmuradov, I.A.; Salamov, A.A. Identification of promoter regions and regulatory sites. Computational biology of transcription factor binding. Methods Mol. Biol. 2010, 674, 57–83. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Zakrajsek, B.A. Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 2000, 46, 69–81. [Google Scholar] [CrossRef]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G.; Wajs-Bonikowska, A.; Bonikowski, R.; Sienkiewicz, M. The Increase of triterpene saponin production induced by trans-anethole in hairy root cultures of Panax quinquefolium. Molecules 2018, 23, 2674. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochan, E.; Balcerczak, E.; Szymczyk, P.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Szymańska, G. Abscisic Acid Regulates the 3-Hydroxy-3-methylglutaryl CoA Reductase Gene Promoter and Ginsenoside Production in Panax quinquefolium Hairy Root Cultures. Int. J. Mol. Sci. 2019, 20, 1310. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20061310
Kochan E, Balcerczak E, Szymczyk P, Sienkiewicz M, Zielińska-Bliźniewska H, Szymańska G. Abscisic Acid Regulates the 3-Hydroxy-3-methylglutaryl CoA Reductase Gene Promoter and Ginsenoside Production in Panax quinquefolium Hairy Root Cultures. International Journal of Molecular Sciences. 2019; 20(6):1310. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20061310
Chicago/Turabian StyleKochan, Ewa, Ewa Balcerczak, Piotr Szymczyk, Monika Sienkiewicz, Hanna Zielińska-Bliźniewska, and Grażyna Szymańska. 2019. "Abscisic Acid Regulates the 3-Hydroxy-3-methylglutaryl CoA Reductase Gene Promoter and Ginsenoside Production in Panax quinquefolium Hairy Root Cultures" International Journal of Molecular Sciences 20, no. 6: 1310. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20061310