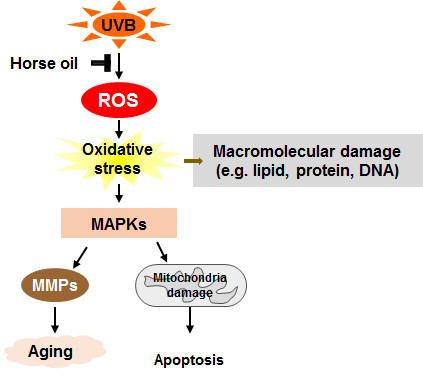
Horse Oil Mitigates Oxidative Damage to Human HaCaT Keratinocytes Caused by Ultraviolet B Irradiation
Abstract
:1. Introduction
2. Results
2.1. Analysis of Horse Oil Composition
2.2. Effect of Horse Oil on UVB-Induced ROS Generation
2.3. Effect of Horse Oil on UVB-Induced Macromolecular Damage
2.4. Effect of Horse Oil on UVB-Induced Apoptosis and MAPK Signaling
2.5. Effect of Horse Oil on UVB Absorption
2.6. Effect of Horse Oil on UVB-Induced MMP Expression and Activation
3. Discussion
4. Materials and Methods
4.1. Preparation of Horse Oil
4.2. Fatty Acid Analysis of Horse Oil
4.3. Cell Culture and UVB Radiation
4.4. Cell Viability Assay
4.5. Detection of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radicals
4.6. Detection of Intracellular ROS
4.7. Lipid Peroxidation Assay
4.8. Protein Carbonyl Formation
4.9. Single-Cell Gel Electrophoresis (Comet Assay)
4.10. Detection of 8-Oxoguanine (8-oxoG)
4.11. CPD Detection
4.12. Mitochondrial Membrane Potential (Δψm) Analysis
4.13. DNA Fragmentation Detection
4.14. Nuclear Staining with Hoechst 33342
4.15. Western Blot Analysis
4.16. UV/visible Light Absorption Analysis
4.17. MMP-1 Activity Assay
4.18. Chromatin Immunoprecipitation (ChIP) Assay
4.19. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AP-1 | Activation protein 1 |
| CPD | Cyclobutane pyrimidine dimer |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DPPP | Diphenyl-1-pyrenylphosphine |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| H2O2 | Hydrogen peroxide |
| JNK1/2 | c-Jun N-terminal kinase 1/2 |
| MMP | Matrix metalloproteinase |
| MTT | Thiazolyl blue tetrazolium bromide |
| NAC | N-acetylcysteine |
| ROS | Reactive oxygen species |
| UV | Ultraviolet |
References
- Brooker, E.G.; Shorland, F.B. Studies on the composition of horse oil. 1. Composition of horse oil in relation to the depot fats of other pasture-fed animals. Biochem. J. 1950, 46, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Al-Shudiefat, A.A.; Sharma, A.K.; Bagchi, A.K.; Dhingra, S.; Singal, P.K. Oleic acid mitigates TNF-α-induced oxidative stress in rat cardiomyocytes. Mol. Cell. Biochem. 2013, 372, 75–82. [Google Scholar] [CrossRef]
- Guzmán, D.C.; Brizuela, N.O.; Herrera, M.O.; Olguín, H.J.; García, E.H.; Peraza, A.V.; Mejía, G.B. Oleic acid protects against oxidative stress exacerbated by cytarabine and doxorubicin in rat brain. Anticancer Agents Med. Chem. 2016, 16, 1491–1495. [Google Scholar] [CrossRef]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Tröscher, A.; Bernabucci, U. Comparison between conjugated linoleic acid and essential fatty acids in preventing oxidative stress in bovine mammary epithelial cells. J. Dairy Sci. 2017, 100, 2299–2309. [Google Scholar] [CrossRef]
- Kim, H.; Youn, K.; Yun, E.Y.; Hwang, J.S.; Jeong, W.S.; Ho, C.T.; Jun, M. Oleic acid ameliorates Aβ-induced inflammation by downregulation of COX-2 and iNOS via NFκB signaling pathway. J. Funct. Foods 2015, 14, 1–11. [Google Scholar] [CrossRef]
- Kim, H.H.; Shin, C.M.; Park, C.H.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J. Lipid Res. 2005, 46, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleszczyński, K.; Zillikens, D.; Fischer, T.W. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J. Pineal Res. 2016, 61, 187–197. [Google Scholar] [PubMed]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and its metabolites ameliorate UVR-induced mitochondrial oxidative stress in human MNT-1 melanoma cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem. Photobiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef]
- Moore, C.; Cevikbas, F.; Pasolli, H.A.; Chen, Y.; Kong, W.; Kempkes, C.; Parekh, P.; Lee, S.H.; Kontchou, N.A.; Yeh, I.; et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E3225–E3234. [Google Scholar] [CrossRef] [Green Version]
- Kleszczyński, K.; Zwicker, S.; Tukaj, S.; Kasperkiewicz, M.; Zillikens, D.; Wolf, R.; Fischer, T.W. Melatonin compensates silencing of heat shock protein 70 and suppresses ultraviolet radiation-induced inflammation in human skin ex vivo and cultured keratinocytes. J. Pineal Res. 2015, 58, 117–126. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet radiation-induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef]
- Balupillai, A.; Nagarajan, R.P.; Ramasamy, K.; Govindasamy, K.; Muthusamy, G. Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signaling in human dermal fibroblasts and mouse skin. Toxicol. Appl. Pharmacol. 2018, 352, 87–96. [Google Scholar] [CrossRef]
- Tong, L.; Wu, S. The Mechanisms of carnosol in chemoprevention of ultraviolet B-light-induced non-melanoma skin cancer formation. Sci. Rep. 2018, 8, 3574. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Jarrett, S.G.; Lee, E.F.; Duprey, C.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci. Rep. 2017, 7, 1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.I.; Jin, S.G.; Pfeifer, G.P. Formation of cyclobutane pyrimidine dimers at dipyrimidines containing 5-hydroxymethylcytosine. Photochem. Photobiol. Sci. 2013, 12, 1409–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, E.; Lee, D.G.; Park, S.H.; Oh, M.S.; Kim, S.Y. Coriander leaf extract exerts antioxidant activity and protects against UVB-induced photoaging of skin by regulation of procollagen type I and MMP-1 expression. J. Med. Food 2014, 17, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Cho, B.O.; Che, D.N.; Shin, J.Y.; Kang, H.J.; Kim, J.H.; Kim, H.Y.; Cho, W.G.; Jang, S.I. Ameliorative effects of Diospyros lotus leaf extract against UVB-induced skin damage in BALB/c mice. Biomed. Pharmacother. 2017, 95, 264–274. [Google Scholar] [CrossRef]
- Misawa, E.; Tanaka, M.; Saito, M.; Nabeshima, K.; Yao, R.; Yamauchi, K.; Abe, F.; Yamamoto, Y.; Furukawa, F. Protective effects of Aloe sterols against UVB-induced photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2017, 33, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Seo, S.A.; Lin, P.; Yi, T.H. Thymus vulgaris alleviates UVB irradiation induced skin damage via inhibition of MAPK/AP-1 and activation of Nrf2-ARE antioxidant system. J. Cell. Mol. Med. 2017, 21, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Nam, M.H.; Lee, K.W. Plantamajoside inhibits UVB and advanced glycation end products-induced MMP-1 expression by suppressing the MAPK and NF-κB pathways in HaCaT cells. Photochem. Photobiol. 2016, 92, 708–719. [Google Scholar] [CrossRef]
- Oh, M.C.; Piao, M.J.; Fernando, P.M.; Han, X.; Hewage, S.R.K.M.; Park, J.E.; Ko, M.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Baicalein protects human skin cells against ultraviolet B-induced oxidative stress. Biomol. Ther. 2016, 24, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Perkins, C.L.; Fang, G.; Kim, C.N.; Bhalla, K.N. The role of Apaf-1, caspase-9, and bid proteins in etoposide- or paclitaxel-induced mitochondrial events during apoptosis. Cancer Res. 2000, 60, 1645–1653. [Google Scholar]
- Afnan, Q.; Kaiser, P.J.; Rafiq, R.A.; Nazir, L.A.; Bhushan, S.; Bhardwaj, S.C.; Sandhir, R.; Tasduq, S.A. Glycyrrhizic acid prevents ultraviolet-B-induced photodamage: a role for mitogen-activated protein kinases, nuclear factor kappa B and mitochondrial apoptotic pathway. Exp. Dermatol. 2016, 25, 440–446. [Google Scholar] [CrossRef]
- Moriyama, M.; Moriyama, H.; Uda, J.; Kubo, H.; Nakajima, Y.; Goto, A.; Morita, T.; Hayakawa, T. BNIP3 upregulation via stimulation of ERK and JNK activity is required for the protection of keratinocytes from UVB-induced apoptosis. Cell Death Dis. 2017, 8, e2576. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Hou, H.; Fan, Y.; Wang, S.; Chen, Q.; Si, L.; Li, B. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J. Photochem. Photobiol. B 2016, 165, 34–41. [Google Scholar] [CrossRef]
- Park, H.C.; Jung, T.K.; Kim, M.J.; Yoon, K.S. Protective effect of Cornus walteri Wangerin leaf against UVB irradiation induced photoaging in human reconstituted skin. J. Ethnopharmacol. 2016, 193, 445–449. [Google Scholar] [CrossRef]
- Hong, S.; Wang, D.; Horton, J.R.; Zhang, X.; Speck, S.H.; Blumenthal, R.M.; Cheng, X. Methyl-dependent and spatial-specific DNA recognition by the orthologous transcription factors human AP-1 and Epstein-Barr virus Zta. Nucleic Acids Res. 2017, 45, 2503–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.B.; Yun, J.G.; Hwang, J.K. Protective effects of standardized Siegesbeckia glabrescens extract and its active compound kirenol against UVB-induced photoaging through inhibition of MAPK/NF-κB pathways. J. Microbiol. Biotechnol. 2017, 27, 242–250. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.; Chung, H.T.; Pae, H.O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar] [PubMed]
- Jung, Y.; Kim, J.C.; Choi, Y.; Lee, S.; Kang, K.S.; Kim, Y.K.; Kim, S.N. Eupatilin with PPARα agonistic effects inhibits TNFα-induced MMP signaling in HaCaT cells. Biochem. Biophys. Res. Commun. 2017, 493, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Amano, S. Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures. Exp. Dermatol. 2016, 25, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, D.; You, L.; Ren, Y.; Wen, L.; Zheng, G.; Li, C. Protective effect of polysaccharides from Sargassum fusiforme against UVB-induced oxidative stress in HaCaT human keratinocytes. J. Funct. Foods 2017, 36, 332–340. [Google Scholar] [CrossRef]
- Goffin, L.; Seguin-Estévez, Q.; Alvarez, M.; Reith, W.; Chizzolini, C. Transcriptional regulation of matrix metalloproteinase-1 and collagen 1A2 explains the anti-fibrotic effect exerted by proteasome inhibition in human dermal fibroblasts. Arthritis Res. Ther. 2010, 12, R73. [Google Scholar] [CrossRef]
- Beeharry, N.; Lowe, J.E.; Hernandez, A.R.; Chambers, J.A.; Fucassi, F.; Cragg, P.J.; Green, M.H.; Green, I.C. Linoleic acid and antioxidants protect against DNA damage and apoptosis induced by palmitic acid. Mutat. Res. 2003, 530, 27–33. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Liu, J.; Cheng, J.; Lu, Y.; Chen, Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- Park, M.A.; Sim, M.J.; Kim, Y.C. Anti-photoaging effects of Angelica acutiloba root ethanol extract in human dermal fibroblasts. Toxicol. Res. 2017, 33, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Chan, C.P.; Chen, Y.J.; Hsien, H.C.; Chang, Y.C.; Yeung, S.Y.; Jeng, P.Y.; Cheng, R.H.; Hahn, L.J.; Jeng, J.H. Areca nut components stimulate ADAM17, IL-1α, PGE2 and 8-isoprostane production in oral keratinocyte: role of reactive oxygen species, EGF and JAK signaling. Oncotarget 2016, 7, 16879–16894. [Google Scholar] [PubMed] [Green Version]
- Lee, H.S.; Lee, S.J.; Yu, H.J.; Lee, J.H.; Cho, H.Y. Fermentation with Lactobacillus enhances the preventive effect of garlic extract on high fat diet-induced hepatic steatosis in mice. J. Funct. Foods 2017, 30, 125–133. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Kim, K.C.; Cha, J.W.; Lee, N.H.; Hyun, J.W. Phloroglucinol enhances the repair of UVB radiation-induced DNA damage via promotion of the nucleotide excision repair system in vitro and in vivo. DNA Repair 2015, 28, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Piao, M.J.; Kang, K.A.; Shilnikova, K.; Hyun, Y.J.; Oh, S.K.; Jeong, Y.J.; Chae, S.; Hyun, J.W. A benzylideneacetophenone derivative induces apoptosis of radiation-resistant human breast cancer cells via oxidative stress. Biomol. Ther. 2017, 25, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Shilnikova, K.; Park, J.E.; Hyun, Y.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Hyun, J.W. The red algae compound 3-bromo-4,5-dihydroxybenzaldehyde protects human keratinocytes on oxidative stress-related molecules and pathways activated by UVB irradiation. Mar. Drugs 2017, 15, 268. [Google Scholar] [CrossRef] [PubMed]







| Fatty Acid | HO-I (%) | HO-II (%) |
|---|---|---|
| C10:0 Capric acid | 0.1 | |
| C12:0 Lauric acid | 0.2 | 2.1 |
| C14:0 Myristic acid | 3.5 | 5.7 |
| C16:0 Palmitic acid | 23.8 | 29.4 |
| C18:0 Stearic acid | 2.8 | 4.8 |
| Saturated fatty acid | 30.3 | 42.1 |
| C14:1 Myristoleic acid | 0.4 | 0.3 |
| C16:1 Palmitoleic acid | 7.9 | 5.2 |
| C18:1 Oleic acid | 37.7 | 32.5 |
| C18:2 Linoleic acid | 19.7 | 15.0 |
| C18:3 Linolenic acid | 2.5 | 3.1 |
| C20:1 Gadoleic acid | 0.7 | 0.7 |
| C20:2 Eicosadienoic acid | 0.4 | 0.3 |
| Unsaturated fatty acid | 69.3 | 57.1 |
| Unknown | 0.4 | 0.8 |
| Total | 100 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, M.J.; Kang, K.A.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Kim, B.S.; Hyun, J.W. Horse Oil Mitigates Oxidative Damage to Human HaCaT Keratinocytes Caused by Ultraviolet B Irradiation. Int. J. Mol. Sci. 2019, 20, 1490. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20061490
Piao MJ, Kang KA, Zhen AX, Kang HK, Koh YS, Kim BS, Hyun JW. Horse Oil Mitigates Oxidative Damage to Human HaCaT Keratinocytes Caused by Ultraviolet B Irradiation. International Journal of Molecular Sciences. 2019; 20(6):1490. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20061490
Chicago/Turabian StylePiao, Mei Jing, Kyoung Ah Kang, Ao Xuan Zhen, Hee Kyoung Kang, Young Sang Koh, Bong Seok Kim, and Jin Won Hyun. 2019. "Horse Oil Mitigates Oxidative Damage to Human HaCaT Keratinocytes Caused by Ultraviolet B Irradiation" International Journal of Molecular Sciences 20, no. 6: 1490. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20061490