The Role of Nitric Oxide in Regulating Intestinal Redox Status and Intestinal Epithelial Cell Functionality
Abstract
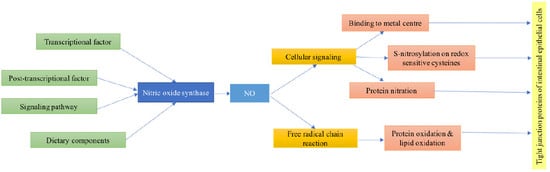
:1. Introduction
2. Regulation of Nitric Oxide Synthase Expression
3. NO Regulation of Intestinal Tight Junction Function
4. Effect of Nitric Oxide on Intestinal Epithelial Tight Junctions: Mechanism of Action
4.1. The Involvement of Nitric Oxide in Cellular Signaling Activity
4.1.1. Binding to Metal Centers
4.1.2. S-Nitrosylation on Redox Sensitive Cysteines of Susceptible Proteins
4.1.3. Reactions of NO with Proteins that Affect Cell Signaling
4.2. Effect of NO on Protein Oxidation and Interference with Lipid and Protein Oxidation Reactions
5. Conclusions
Funding
Conflicts of Interest
References
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Knowles, R.G.; Moncada, S. Nitric oxide synthases in mammals. Biochem. J. 1994, 298 Pt 2, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Marletta, M.A. Nitric oxide synthase: Aspects concerning structure and catalysis. Cell 1994, 78, 927–930. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Tsuji, H.; Onoda, M. Nitric oxide attenuates hydrogen peroxide-induced barrier disruption and protein tyrosine phosphorylation in monolayers of intestinal epithelial cell. Biochim. Biophys. Acta 2007, 1773, 794–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gow, A.J.; Farkouh, C.R.; Munson, D.A.; Posencheg, M.A.; Ischiropoulos, H. Biological significance of nitric oxide-mediated protein modifications. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L262–L268. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, I. Nitric oxide mediated redox regulation of protein homeostasis. Cell. Signal. 2019, 53, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric Oxide Production and Signaling in Inflammation. Curr. Drug Target Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Salzman, A.L.; Menconi, M.J.; Unno, N.; Ezzell, R.M.; Casey, D.M.; Gonzalez, P.K.; Fink, M.P. Nitric oxide dilates tight junctions and depletes ATP in cultured Caco-2BBe intestinal epithelial monolayers. Am. J. Physio. Gastrointest. Liver Physiol. 1995, 268, G361–G373. [Google Scholar] [CrossRef] [PubMed]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the Role of TNF-α and IFN-γ Activation on the Dynamics of iNOS Gene Expression in LPS Stimulated Macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef] [PubMed]
- Vilar, A.; de Lemos, L.; Patraca, I.; Martínez, N.; Folch, J.; Junyent, F.; Verdaguer, E.; Pallàs, M.; Auladell, C.; Camins, A. Melatonin suppresses nitric oxide production in glial cultures by pro-inflammatory cytokines through p38 MAPK inhibition. Free Radic. Res. 2014, 48, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Witthöft, T.; Eckmann, L.; Kim, J.M.; Kagnoff, M.F. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1998, 275, G564–G571. [Google Scholar] [CrossRef] [PubMed]
- Salzman, A.L.; Eaves Pyles, T.; Linn, S.C.; Denenberg, A.G.; Szabó, C. Bacterial induction of inducible nitric oxide synthase in cultured human intestinal epithelial cells. Gastroenterology 1998, 114, 93–102. [Google Scholar] [CrossRef]
- Sanongkiet, S.; Ponnikorn, S.; Udomsangpetch, R.; Tungpradabkul, S. Burkholderia pseudomallei rpoS mediates iNOS suppression in human hepatocyte (HC04) cells. FEMS Microbiol. Lett. 2016, 363, fnw161. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Y.; Lu, J.; Liu, M.; Dai, B.; Miao, J.; Yin, Y. Variant innate immune responses of mammary epithelial cells to challenge by Staphylococcus aureus, Escherichia coli and the regulating effect of taurine on these bioprocesses. Free Radic. Biol. Med. 2016, 96, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, T.; Tuominen, R.K.; Moilanen, E. Protein kinase C and its inhibitors in the regulation of inflammation: Inducible nitric oxide synthase as an example. Basic Clin. Pharmacol. Toxicol. 2014, 114, 37–43. [Google Scholar] [CrossRef]
- Lakshmanan, J.; Zhang, B.; Nweze, I.C.; Du, Y.; Harbrecht, B.G. Glycogen synthase kinase 3 regulates IL-1β mediated iNOS expression in hepatocytes by down-regulating c-Jun. J. Cell. Biochem. 2015, 116, 133–141. [Google Scholar] [CrossRef]
- Peng, X.-X.; Zhang, S.-H.; Wang, X.-L.; Ye, T.-J.; Li, H.; Yan, X.-F.; Wei, L.; Wu, Z.-P.; Hu, J.; Zou, C.-P.; et al. Panax Notoginseng flower saponins (PNFS) inhibit LPS-stimulated NO overproduction and iNOS gene overexpression via the suppression of TLR4-mediated MAPK/NF-kappa B signaling pathways in RAW264.7 macrophages. Chin. Med. 2015, 10, 15. [Google Scholar] [CrossRef]
- Bak, J.; Je, N.K.; Chung, H.Y.; Yokozawa, T.; Yoon, S.; Moon, J.-O. Oligonol Ameliorates CCl₄-Induced Liver Injury in Rats via the NF-Kappa B and MAPK Signaling Pathways. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Ko, H.M.; Lee, S.H.; Bang, M.; Kim, K.C.; Jeon, S.J.; Park, Y.-M.; Han, S.-H.; Kim, H.Y.; Lee, J.; Shin, C.Y. Tyrosine kinase Fyn regulates iNOS expression in LPS-stimulated astrocytes via modulation of ERK phosphorylation. Biochem. Biophys. Res. Commun. 2018, 495, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, M.E.; Lee, J.S. Inhibitory effects of extract from G. lanceolata on LPS-induced production of nitric oxide and IL-1β via down-regulation of MAPK in macrophages. Appl. Biochem. Biotechnol. 2015, 175, 657–665. [Google Scholar] [CrossRef]
- Chen, C.C.; Wang, J.K.; Chen, W.C.; Lin, S.B. Protein kinase C eta mediates lipopolysaccharide-induced nitric-oxide synthase expression in primary astrocytes. J. Biol. Chem. 1998, 273, 19424–19430. [Google Scholar] [CrossRef]
- Marczin, N.; Papapetropoulos, A.; Catravas, J.D. Tyrosine kinase inhibitors suppress endotoxin- and IL-1 beta-induced NO synthesis in aortic smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 1993, 265, H1014–H1018. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.R.; Feinstein, D.L.; Shen, Q.; Bhat, A.N. p38 MAPK-mediated Transcriptional Activation of Inducible Nitric-oxide Synthase in Glial Cells. J. Biol. Chem. 2002, 277, 29584–29592. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Guerra, M.J.; Castrillo, A.; Martín-Sanz, P.; Boscá, L. Negative regulation by protein tyrosine phosphatase of IFN-gamma-dependent expression of inducible nitric oxide synthase. J. Immunol. 1999, 162, 6776–6783. [Google Scholar] [PubMed]
- Díaz-Guerra, M.J.; Castrillo, A.; Martín-Sanz, P.; Boscá, L. Negative regulation by phosphatidylinositol 3-kinase of inducible nitric oxide synthase expression in macrophages. J. Immunol. 1999, 162, 6184–6190. [Google Scholar]
- Kleinert, H.; Euchenhofer, C.; Ihrig-Biedert, I.; Förstermann, U. In Murine 3T3 Fibroblasts, Different Second Messenger Pathways Resulting in the Induction of NO Synthase II (iNOS) Converge in the Activation of Transcription Factor NF-B. J. Biol. Chem. 1996, 271, 6039–6044. [Google Scholar] [CrossRef]
- Marks-Konczalik, J.; Chu, S.C.; Moss, J. Cytokine-mediated Transcriptional Induction of the Human Inducible Nitric Oxide Synthase Gene Requires Both Activator Protein 1 and Nuclear Factor κB-binding Sites. J. Biol. Chem. 1998, 273, 22201–22208. [Google Scholar] [CrossRef]
- Baig, M.S.; Zaichick, S.V.; Mao, M.; de Abreu, A.L.; Bakhshi, F.R.; Hart, P.C.; Saqib, U.; Deng, J.; Chatterjee, S.; Block, M.L.; et al. NOS1-derived nitric oxide promotes NF-κB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J. Exp. Med. 2015, 212, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Braverman, J.; Stanley, S.A. Nitric Oxide Modulates Macrophage Responses to Mycobacterium tuberculosis Infection through Activation of HIF-1α and Repression of NF-κB. J. Immunol. 2017, 199, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- De Vera, M.E.; Shapiro, R.A.; Nussler, A.K.; Mudgett, J.S.; Simmons, R.L.; Morris, S.M.; Billiar, T.R.; Geller, D.A. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: Initial analysis of the human NOS2 promoter. Proc. Natl. Acad. Sci. USA 1996, 93, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Laubach, V.E.; Zhang, C.X.; Russell, S.W.; Murphy, W.J.; Sherman, P.A. Analysis of expression and promoter function of the human inducible nitric oxide synthase gene in DLD-1 cells and monkey hepatocytes. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1997, 1351, 287–295. [Google Scholar] [CrossRef]
- Linn, S.C.; Morelli, P.J.; Edry, I.; Cottongim, S.E.; Szabo, C.; Salzman, A.L. Transcriptional regulation of human inducible nitric oxide synthase gene in an intestinal epithelial cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 272, G1499–G1508. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kar, S.; Basu Ball, W.; Ghosh, K.; Das, P.K. The curative effect of fucoidan on visceral leishmaniasis is mediated by activation of MAP kinases through specific protein kinase C isoforms. Cell. Mol. Immunol. 2014, 11, 263–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.-H.; Kim, M.-S.; Le, M.-Q.; Song, Y.-S.; Bak, Y.; Ryu, H.-W.; Oh, S.-R.; Yoon, D.-Y. Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and NF-ĸB signaling. Phytomedicine 2017, 24, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zong, C.; Gao, Y.; Cai, R.; Fang, L.; Lu, J.; Liu, F.; Qi, Y. Curcumol exhibits anti-inflammatory properties by interfering with the JNK-mediated AP-1 pathway in lipopolysaccharide-activated RAW264.7 cells. Eur. J. Pharmacol. 2014, 723, 339–345. [Google Scholar] [CrossRef]
- Yang, C.; Yu, L.; Kong, L.; Ma, R.; Zhang, J.; Zhu, Q.; Zhu, J.; Hao, D. Pyrroloquinoline quinone (PQQ) inhibits lipopolysaccharide induced inflammation in part via downregulated NF-κB and p38/JNK activation in microglial and attenuates microglia activation in lipopolysaccharide treatment mice. PLoS ONE 2014, 9, e109502. [Google Scholar] [CrossRef]
- Li, L.; Sapkota, M.; Kim, S.-W.; Soh, Y. Herbacetin inhibits inducible nitric oxide synthase via JNK and nuclear factor-κB in LPS-stimulated RAW264.7 cells. Eur. J. Pharmacol. 2015, 765, 115–123. [Google Scholar] [CrossRef]
- Vodovotz, Y.; Bogdan, C.; Paik, J.; Xie, Q.W.; Nathan, C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J. Exp. Med. 1993, 178, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Cabrié, A.; Guittet, O.; Tomasini, R.; Vincendeau, P.; Lepoivre, M. Crosstalk between TAp73 and TGF-β in fibroblast regulates iNOS expression and Nrf2-dependent gene transcription. Free Radic. Biol. Med. 2019, 134, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Hämäläinen, M.; Kankaanranta, H.; Moilanen, E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol. Pharmacol. 2002, 62, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, X.-F.; Cao, K.; Ding, J.; Kang, X.; Guan, W.-X.; Ding, Y.-T.; Liu, B.-R.; Du, J.-F. Dexamethasone-Induced Myeloid-Derived Suppressor Cells Prolong Allo Cardiac Graft Survival through iNOS- and Glucocorticoid Receptor-Dependent Mechanism. Front. Immunol. 2018, 9, 282. [Google Scholar] [CrossRef]
- Moreira, D.R. Inhibition of Nitric Oxide Synthesis by Dexamethasone Increases Survival Rate In Plasmodium berghei-Infected Mice. bioRxiv 2018, 497966. [Google Scholar] [CrossRef]
- Pérez-Sala, D.; Cernuda-Morollón, E.; Díaz-Cazorla, M.; Rodríguez-Pascual, F.; Lamas, S. Posttranscriptional regulation of human iNOS by the NO/cGMP pathway. Am. J. Physiol. Renal Physiol. 2001, 280, F466–F473. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Zhang, F.; Ying, C.J.; Chen, J.; Chen, L.; Dong, J.; Shi, Y.; Tang, M.; Hu, X.T.; Pan, Z.H.; et al. Inhibition of iNOS alleviates cognitive deficits and depression in diabetic mice through downregulating the NO/sGC/cGMP/PKG signal pathway. Behav. Brain Res. 2017, 322, 70–82. [Google Scholar] [CrossRef]
- Geng, Y.; Lotz, M. Increased intracellular Ca2+ selectively suppresses IL-1-induced NO production by reducing iNOS mRNA stability. J. Cell Biol. 1995, 129, 1651–1657. [Google Scholar] [CrossRef] [Green Version]
- Kunz, D.; Muhl, H.; Walker, G.; Pfeilschifter, J. Two distinct signaling pathways trigger the expression of inducible nitric oxide synthase in rat renal mesangial cells. Proc. Natl. Acad. Sci. USA 1994, 91, 5387–5391. [Google Scholar] [CrossRef]
- Oddis, C.V.; Simmons, R.L.; Hattler, B.G.; Finkel, M.S. cAMP enhances inducible nitric oxide synthase mRNA stability in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 1995, 269, H2044–H2050. [Google Scholar] [CrossRef]
- Linscheid, P.; Schaffner, A.; Schoedon, G. Modulation of Inducible Nitric Oxide Synthase mRNA Stability by Tetrahydrobiopterin in Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 1998, 243, 137–141. [Google Scholar] [CrossRef]
- McNeill, E.; Crabtree, M.J.; Sahgal, N.; Patel, J.; Chuaiphichai, S.; Iqbal, A.J.; Hale, A.B.; Greaves, D.R.; Channon, K.M. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic. Biol. Med. 2015, 79, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Guo, Q.; Duan, X.; Xu, Z.; Wang, Q. l-arginine inhibited apoptosis of fish leukocytes via regulation of NF-κB-mediated inflammation, NO synthesis, and anti-oxidant capacity. Biochimie 2019, 158, 62–72. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, X.; He, Q.; Li, J.; Lu, J.; Zou, X. L-arginine stimulates CAT-1-mediated arginine uptake and regulation of inducible nitric oxide synthase for the growth of chick intestinal epithelial cells. Mol. Cell. Biochem. 2015, 399, 229–236. [Google Scholar] [CrossRef]
- Pahan, K.; Namboodiri, A.M.; Sheikh, F.G.; Smith, B.T.; Singh, I. Increasing cAMP attenuates induction of inducible nitric-oxide synthase in rat primary astrocytes. J. Biol. Chem. 1997, 272, 7786–7791. [Google Scholar] [CrossRef]
- Paul, A.; Doherty, K.; Plevin, R. Differential regulation by protein kinase C isoforms of nitric oxide synthase induction in RAW 264.7 macrophages and rat aortic smooth muscle cells. Br. J. Pharmacol. 1997, 120, 940–946. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.-C.; Chou, C.-F.; Lin, W.-W. Pyrimidinoceptor-mediated Potentiation of Inducible Nitric-oxide Synthase Induction in J774 Macrophages. J. Biol. Chem. 1998, 273, 29754–29763. [Google Scholar] [CrossRef]
- Bereta, M.; Bereta, J.; Georgoff, I.; Coffman, F.D.; Cohen, S.; Cohen, M.C. Methylxanthines and Calcium-Mobilizing Agents Inhibit the Expression of Cytokine-Inducible Nitric Oxide Synthase and Vascular Cell Adhesion Molecule-1 in Murine Microvascular Endothelial Cells. Exp. Cell Res. 1994, 212, 230–242. [Google Scholar] [CrossRef]
- Chen, B.C.; Chen, Y.H.; Lin, W.W. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology 1999, 97, 124–129. [Google Scholar] [CrossRef]
- Kristof, A.S.; Marks-Konczalik, J.; Moss, J. Mitogen-activated protein kinases mediate activator protein-1-dependent human inducible nitric-oxide synthase promoter activation. J. Biol. Chem. 2001, 276, 8445–8452. [Google Scholar] [CrossRef]
- Cho, M.K.; Suh, S.H.; Kim, S.G. JunB/AP-1 and NF-κB-Mediated Induction of Nitric Oxide Synthase by Bovine Type I Collagen in Serum-Stimulated Murine Macrophages. Nitric Oxide 2002, 6, 319–332. [Google Scholar] [CrossRef]
- Guan, Z.; Baier, L.D.; Morrison, A.R. p38 Mitogen-activated Protein Kinase Down-regulates Nitric Oxide and Up-regulates Prostaglandin E 2Biosynthesis Stimulated by Interleukin-1β. J. Biol. Chem. 1997, 272, 8083–8089. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Olejnik, A.; Kowalska, K.; Olkowicz, M.; Juzwa, W.; Dembczynski, R.; Schmidt, M. A Gastrointestinally Digested Ribes nigrum L. Fruit Extract Inhibits Inflammatory Response in a Co-culture Model of Intestinal Caco-2 Cells and RAW264.7 Macrophages. J. Agric. Food Chem. 2016, 64, 7710–7721. [Google Scholar] [CrossRef]
- Olejnik, A.; Kowalska, K.; Kidoń, M.; Czapski, J.; Rychlik, J.; Olkowicz, M.; Dembczyński, R. Purple carrot anthocyanins suppress lipopolysaccharide-induced inflammation in the co-culture of intestinal Caco-2 and macrophage RAW264.7 cells. Food Funct. 2016, 7, 557–564. [Google Scholar] [CrossRef]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.; Bucknall, M.; Arcot, J. Effect of Different Anthocyanidin Glucosides on Lutein Uptake by Caco-2 Cells, and Their Combined Activities on Anti-Oxidation and Anti-Inflammation In Vitro and Ex Vivo. Molecules 2018, 23, 2035. [Google Scholar] [CrossRef]
- Calvello, R.; Aresta, A.; Trapani, A.; Zambonin, C.; Cianciulli, A.; Salvatore, R.; Clodoveo, M.L.; Corbo, F.; Franchini, C.; Panaro, M.A. Bovine and soybean milk bioactive compounds: Effects on inflammatory response of human intestinal Caco-2 cells. Food Chem. 2016, 210, 276–285. [Google Scholar] [CrossRef]
- Meng, Q.; Cooney, M.; Yepuri, N.; Cooney, R.N. L-arginine attenuates Interleukin-1β (IL-1β) induced Nuclear Factor Kappa-Beta (NF-κB) activation in Caco-2 cells. PLoS ONE 2017, 12, e0174441. [Google Scholar] [CrossRef]
- Chen, X.-M.; Kitts, D.D. Evidence for inhibition of nitric oxide and inducible nitric oxide synthase in Caco-2 and RAW 264.7 cells by a Maillard reaction product [5-(5,6-dihydro-4H-pyridin-3-ylidenemethyl)furan-2-yl]-methanol. Mol. Cell. Biochem. 2015, 406, 205–215. [Google Scholar] [CrossRef]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.P.; Tuberoso, C.I.G.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols -induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef]
- Serreli, G.; Melis, M.P.; Corona, G.; Deiana, M. Modulation of LPS-induced nitric oxide production in intestinal cells by hydroxytyrosol and tyrosol metabolites: Insight into the mechanism of action. Food Chem. Toxicol. 2019, 125, 520–527. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y. Intestinal anti-inflammatory effects of cinnamon extracts in a co-culture model of intestinal epithelial Caco-2 cells and RAW264.7 macrophages. Appl. Biol. Chem. 2017, 60, 553–561. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Han, X.; Fink, M.P.; Delude, R.L. Proinflammatory Cytokines Cause No??-Dependent and -Independent Changes in Expression and Localization of Tight Junction Proteins in Intestinal Epithelial Cells. Shock 2003, 19, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhang, L.; Forsyth, C.B.; Shaikh, M.; Song, S.; Keshavarzian, A. The Role of miR-212 and iNOS in Alcohol-Induced Intestinal Barrier Dysfunction and Steatohepatitis. Alcohol. Clin. Exp. Res. 2015, 39, 1632–1641. [Google Scholar] [CrossRef]
- Menconi, M.J.; Unno, N.; Smith, M.; Aguirre, D.E.; Fink, M.P. Nitric oxide donor-induced hyperpermeability of cultured intestinal epithelial monolayers: Role of superoxide radical, hydroxyl radical, and peroxynitrite. Biochim. Biophys. Acta 1998, 1425, 189–203. [Google Scholar] [CrossRef]
- Unno, N.; Menconi, M.J.; Smith, M.; Aguirre, D.E.; Fink, M.P. Hyperpermeability of intestinal epithelial monolayers is induced by NO: Effect of low extracellular pH. Am. J. Physiol. 1997, 272, G923–G934. [Google Scholar] [CrossRef]
- Alican, I.; Kubes, P. A critical role for nitric oxide in intestinal barrier function and dysfunction. American J. Physiol. Gastrointest. Liver Physiol. 1996, 270, G225–G237. [Google Scholar] [CrossRef]
- Farhadi, A. The Role of Protein Kinase C Isoforms in Modulating Injury and Repair of the Intestinal Barrier. J. Pharmacol. Exp. Ther. 2005, 316, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Foster, D.C.; Wedel, B.J.; Robinson, S.W.; Garbers, D.L. Mechanisms of regulation and functions of guanylyl cyclases. Rev. Physiol. Biochem. Pharmacol. 1999, 135, 1–39. [Google Scholar]
- Kato, M.; Blanton, R.; Wang, G.-R.; Judson, T.J.; Abe, Y.; Myoishi, M.; Karas, R.H.; Mendelsohn, M.E. Direct binding and regulation of RhoA protein by cyclic GMP-dependent protein kinase Iα. J. Biol. Chem. 2012, 287, 41342–41351. [Google Scholar] [CrossRef]
- Guillemot, L.; Citi, S. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol. Biol. Cell 2006, 17, 3569–3577. [Google Scholar] [CrossRef]
- Rosas-Hernández, R.; Bastián, Y.; Juárez Tello, A.; Ramírez-Saíto, Á.; Escobar García, D.M.; Pozos-Guillén, A.; Mendez, J.A. Alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors modulate the reorganization of F-actin in Bergmann glia cells through the activation of RhoA. J. Neurochem. 2018, 112, 97. [Google Scholar] [CrossRef]
- Sedzinski, J.; Hannezo, E.; Tu, F.; Biro, M.; Wallingford, J.B. RhoA regulates actin network dynamics during apical surface emergence in multiciliated epithelial cells. J. Cell. Sci. 2017, 130, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Stadtman, E.R. Protein Oxidation in Aging and Age-Related Diseases. Ann. N. Y. Acad. Sci. 2006, 928, 22–38. [Google Scholar] [CrossRef]
- Kim, B.; Breton, S. The MAPK/ERK-Signaling Pathway Regulates the Expression and Distribution of Tight Junction Proteins in the Mouse Proximal Epididymis. Biol. Reprod. 2016, 94, 22. [Google Scholar] [CrossRef]
- Lander, H.M.; Hajjar, D.P.; Hempstead, B.L.; Mirza, U.A.; Chait, B.T.; Campbell, S.; Quilliam, L.A. A Molecular Redox Switch on p21 ras. J. Biol. Chem. 1997, 272, 4323–4326. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef]
- Tang, Y.; Clayburgh, D.R.; Mittal, N.; Goretsky, T.; Dirisina, R.; Zhang, Z.; Kron, M.; Ivancic, D.; Katzman, R.B.; Grimm, G.; et al. Epithelial NF-kappaB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. Am. J. Pathol. 2010, 176, 158–167. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, J.; Park, S.-H.; Do, K.H.; Yang, H.; Moon, Y. Pro-inflammatory NF-κB and early growth response gene 1 regulate epithelial barrier disruption by food additive carrageenan in human intestinal epithelial cells. Toxicol. Lett. 2012, 211, 289–295. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M.; Sun, L.; Xiao, W.; Yang, X.; Sun, L.; Zhang, C.; Ma, Y.; Yang, H.; Liu, Y.; et al. Interferon-γ-induced intestinal epithelial barrier dysfunction by NF-κB/HIF-1α pathway. J. Interferon Cytokine Res. 2014, 34, 195–203. [Google Scholar] [CrossRef]
- Jeong, C.H.; Seok, J.S.; Petriello, M.C.; Han, S.G. Arsenic downregulates tight junction claudin proteins through p38 and NF-κB in intestinal epithelial cell line, HT-29. Toxicology 2017, 379, 31–39. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, W.-D.; Jiang, J.; Zhao, J.; Liu, Y.; Zhang, Y.-A.; Zhou, X.-Q.; Feng, L. Dietary choline deficiency and excess induced intestinal inflammation and alteration of intestinal tight junction protein transcription potentially by modulating NF-κB, STAT and p38 MAPK signaling molecules in juvenile Jian carp. Fish Shellfish Immunol. 2016, 58, 462–473. [Google Scholar] [CrossRef]
- Wu, M.; Bian, Q.; Liu, Y.; Fernandes, A.; Taylor, A.; Pereira, P.; Shang, F. Sustained oxidative stress inhibits NF-κB activation partially via inactivating the proteasome. Free Radic. Biol. Med. 2009, 46, 62–69. [Google Scholar] [CrossRef]
- Matthews, J.R.; Botting, C.H.; Panico, M.; Morris, H.R.; Hay, R.T. Inhibition of NF- B DNA Binding by Nitric Oxide. Nucleic Acids Res. 1996, 24, 2236–2242. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Kitts, D.D. Amelioration of oxidative stress in Caco-2 cells treated with pro-inflammatory proteins by chlorogenic acid isomers via activation of the Nrf2-Keap1 signaling pathway. J. Agric. Food Chem. 2018, 66, 11008–11017. [Google Scholar] [CrossRef]
- Brennan, M.-L.; Wu, W.; Fu, X.; Shen, Z.; Song, W.; Frost, H.; Vadseth, C.; Narine, L.; Lenkiewicz, E.; Borchers, M.T.; et al. A tale of two controversies: Defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002, 277, 17415–17427. [Google Scholar] [CrossRef]
- Van der Vliet, A.; Eiserich, J.P.; Halliwell, B.; Cross, C.E. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J. Biol. Chem. 1997, 272, 7617–7625. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Y.; Hazen, S.L. Eosinophil peroxidase nitrates protein tyrosyl residues. Implications for oxidative damage by nitrating intermediates in eosinophilic inflammatory disorders. J. Biol. Chem. 1999, 274, 25933–25944. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Cotgreave, I.A.; Gerdes, R.G. Recent Trends in Glutathione Biochemistry—Glutathione–Protein Interactions: A Molecular Link between Oxidative Stress and Cell Proliferation? Biochem. Biophys. Res. Commun. 1998, 242, 1–9. [Google Scholar] [CrossRef]
- Herrlich, P.; Böhmer, F.D. Redox regulation of signal transduction in mammalian cells. Biochem. Pharmacol. 2000, 59, 35–41. [Google Scholar] [CrossRef]
- Rao, R.K.; Li, L.; Baker, R.D.; Baker, S.S.; Gupta, A. Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G332–G340. [Google Scholar] [CrossRef]
- Sun, J.; Steenbergen, C.; Murphy, E. S-Nitrosylation: NO-Related Redox Signaling to Protect Against Oxidative Stress. Antioxid. Redox Signal. 2006, 8, 1693–1705. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Suzuki, H.; Ueda, N.; Takahashi, Y.; Yoshimoto, T. Mammalian Lipoxygenases. In The Eicosanoids; John Wiley & Sons, Ltd.: Chichester, UK, 2004; pp. 53–59. [Google Scholar]
- Spiteller, G. Peroxyl Radicals Are Essential Reagents in the Oxidation Steps of the Maillard Reaction Leading to Generation of Advanced Glycation End Products. Ann. N. Y. Acad. Sci. 2008, 1126, 128–133. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate Contribution of Lipid Oxidation and Maillard Reaction to the Nonenzymatic Food Browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Carlsen, C.U.; Møller, J.K.S.; Skibsted, L.H. Heme-iron in lipid oxidation. Coord. Chem. Rev. 2005, 249, 485–498. [Google Scholar] [CrossRef]
- Gebicki, S.; Gebicki, J.M. Formation of peroxides in amino acids and proteins exposed to oxygen free radicals. Biochem. J. 1993, 289, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Neužil, J.; Gebicki, J.M.; Stocker, R. Radical-induced chain oxidation of proteins and its inhibition by chain-breaking antioxidants. Biochem. J. 1993, 293, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Forsmark-AndrÉe, P.; Dallner, G.; Ernster, L. Endogenous ubiquinol prevents protein modification accompanying lipid peroxidation in beef heart submitochondrial particles. Free Radic. Biol. Med. 1995, 19, 749–757. [Google Scholar] [CrossRef]
- Gardner, H.W. Lipid hydroperoxide reactivity with proteins and amino acids: A review. J. Agric. Food Chem. 1979, 27, 220–229. [Google Scholar] [CrossRef]
- Schaich, K.M.; Karel, M. Free radical reactions of peroxidizing lipids with amino acids and proteins: An ESR study. Lipids 1976, 11, 392–400. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [Green Version]
- Roubal, W.T.; Tappel, A.L. Damage to proteins, enzymes, and amino acids by peroxidizing lipids. Arch. Biochem. Biophys. 1966, 113, 5–8. [Google Scholar] [CrossRef]
- Götz, M.E.; Gerlach, M. Formation of Radicals. In Brain Damage and Repair; Springer: Dordrecht, The Netherlands, 2004; Volume 8, pp. 135–164. [Google Scholar]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Hogg, N.; Parthasarthy, S.; Kalyanaraman, B. Inhibition of low-density lipoprotein oxidation by nitric oxide. Free Radic. Biol. Med. 1993, 15, 495. [Google Scholar] [CrossRef]
- Rubbo, H.; Radi, R.; Trujillo, M.; Telleri, R.; Kalyanaraman, B.; Barnes, S.; Kirk, M.; Freeman, B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994, 269, 26066–26075. [Google Scholar]
- Rubbo, H.; Parthasarathy, S.; Barnes, S.; Kirk, M.; Kalyanaraman, B.; Freeman, B.A. Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: Termination of radical chain propagation reactions and formation of nitrogen-containing oxidized lipid derivatives. Arch. Biochem. Biophys. 1995, 324, 15–25. [Google Scholar] [CrossRef]
- Hayashi, K.; Noguchi, N.; Niki, E. Action of nitric oxide as an antioxidant against oxidation of soybean phosphatidylcholine liposomal membranes. FEBS Lett. 1995, 370, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Goss, S.P.; Hogg, N.; Kalyanaraman, B. The antioxidant effect of spermine NONOate in human low-density lipoprotein. Chem. Res. Toxicol. 1995, 8, 800–806. [Google Scholar] [CrossRef]
- Goss, S.P.; Hogg, N.; Kalyanaraman, B. The effect of nitric oxide release rates on the oxidation of human low density lipoprotein. J. Biol. Chem. 1997, 272, 21647–21653. [Google Scholar] [CrossRef] [PubMed]
- Darley-usmar, V.M.; Hogg, N.; O’leary, V.J.; Wilson, M.T.; Moncada, S. The Simultaneous Generation of Superoxide and Nitric Oxide Can Initiate Lipid Peroxidation in Human Low Density Lipoprotein. Free Radic. Res. Commun. 2009, 17, 9–20. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids 1992, 27, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S. Oxidative Damage and Tyrosine Nitration from Peroxynitrite. Chem. Res. Toxicol. 1996, 9, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Iravani, M.M.; Kashefi, K.; Mander, P.; Rose, S.; Jenner, P. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience 2002, 110, 49–58. [Google Scholar] [CrossRef]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Gow, A.J.; Ischiropoulos, H. Nitric oxide chemistry and cellular signaling. J. Cell. Physiol. 2001, 187, 277–282. [Google Scholar] [CrossRef] [PubMed]


| Dietary Component | Inducer | Cell Type | Description | Ref |
|---|---|---|---|---|
| Olive oil polyphenols | Oxysterols | Caco-2 cells | Oxysterols induced nitric oxide (NO) generation was suppressed by tested compounds | [70] |
| Hydroxytyrosol and tyrosol metabolites | Lipopolysaccharide (LPS) | Caco-2 cells | LPS-induced NO release was inhibited by tested compounds | [71] |
| Cinnamon | LPS | Caco-2 and Raw 264.7 co-culture | LPS-induced NO release was inhibited by tested compound | [72] |
| Gastrointestinal-digested blackcurrant extracts | LPS | Caco-2 and Raw 264.7 co-culture | LPS-induced NO release was inhibited by tested compounds | [63] |
| Purple carrot anthocyanins | LPS | Caco-2 and Raw 264.7 co-culture | LPS-induced NO release was inhibited by tested compounds | [64] |
| Bovine and soybean milk bioactive compounds | LPS | Caco-2 cells | LPS-induced NO release was inhibited by tested compounds | [67] |
| Resveratrol | LPS | Caco-2 cells or SW480 | LPS-induced NO release was inhibited by tested compound | [65] |
| Lutein | Tumor necrosis factor (TNF)-α | Caco-2 cells | TNF-α was suppressed by tested compound | [66] |
| L-arginine | Interleukin (IL)-1β | Caco-2 cells | IL-1β-induced NO release was inhibited by tested compound | [68] |
| Maillard reaction products | IFN-γ + phorbol 12-myristate 13-acetate (PMA) | Caco-2 cells | Induced NO release was inhibited by tested compounds | [69] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, K.; Yu, S.; Kitts, D.D. The Role of Nitric Oxide in Regulating Intestinal Redox Status and Intestinal Epithelial Cell Functionality. Int. J. Mol. Sci. 2019, 20, 1755. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071755
Mu K, Yu S, Kitts DD. The Role of Nitric Oxide in Regulating Intestinal Redox Status and Intestinal Epithelial Cell Functionality. International Journal of Molecular Sciences. 2019; 20(7):1755. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071755
Chicago/Turabian StyleMu, Kaiwen, Shengwu Yu, and David D. Kitts. 2019. "The Role of Nitric Oxide in Regulating Intestinal Redox Status and Intestinal Epithelial Cell Functionality" International Journal of Molecular Sciences 20, no. 7: 1755. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071755