Influence of Cigarette Smoke Inhalation on an Autogenous Onlay Bone Graft Area in Rats with Estrogen Deficiency: A Histomorphometric and Immunohistochemistry Study
Abstract
:1. Introduction
2. Results
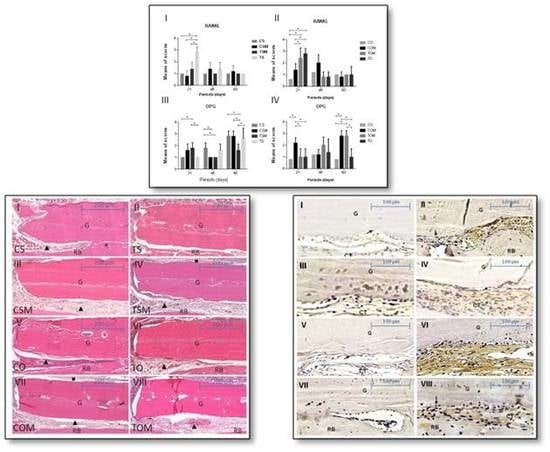
2.1. Descriptive Histology and Statistical Analysis
2.2. Immunohistochemical Assessment
2.2.1. Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL)
2.2.2. OPG
3. Discussion
4. Materials and Methods
4.1. Protocol for Smoking Cigarette Inhalation
4.2. Surgical Procedure
4.3. Euthanasia and Processing of the Sample
4.4. Histomorphometric Analysis
4.5. Immunohistochemistry Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donos, N.; Dereka, X.; Mardas, N. Experimental models for guided bone regeneration in healthy and medically compromised conditions. Periodontol. 2000 2015, 68, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Kostopoulos, L.; Karring, T. Alveolar ridge augmentation by combining autogenous mandibular bone grafts and non-resorbable membranes. An experimental study in the rat. Clin. Oral Implants Res. 2002, 13, 185–191. [Google Scholar] [CrossRef]
- Jardini, M.A.N.; De Marco, A.C.; Lima, L.A. Early healing pattern of autogenous bone grafts with and without e-PTFE membranes: A histomorphometric study in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, W.L.; Reuther, T.; Bloch, W.; Korkmaz, Y.; Fisher, J.H.; Zöller, J.E. Healing of onlay mandibular bone grafts covered with collagen membrane or bovine bone substitutes: A microscopical and immunohistochemical study in the sheep. Int. J. Oral Maxillofac. Surg. 2008, 37, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Luize, D.S.; Bosco, A.F.; Bonfante, S.; Almeida, J.M. Influence of ovariectomy on healing of autogenous bone block grafts in the mandible: A histomorphometric study in na aged rat model. Int. J. Oral Maxillofac. Implants 2008, 23, 207–214. [Google Scholar] [PubMed]
- Lindfors, L.T.; Tervonen, E.A.T.; Sándor, G.K.B.; Ylikontiola, L.P. Guided bone regeneration using a titanium-reinforced ePTFE membrane and particulate autogenous bone: The effect of smoking and membrane exposure. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 825–830. [Google Scholar] [CrossRef]
- Giorgetti, P.O.; César-Neto, J.B.; Ruiz, K.G.S.; Casati, M.Z.; Sallum, E.A.; Nociti Jr, F.H. Cigarette smoke inhalation influences bone healing of postextraction tooth socket: A histometric study in rats. Braz. Dent. J. 2012, 23, 228–234. [Google Scholar] [CrossRef]
- Taguchi, Y.; Amizuka, N.; Nakadate, M.; Ohnishi, H.; Fujii, N.; Oda, K.; Nomura, S.; Maeda, T. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials 2005, 26, 6158–6166. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided bone regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Elgali, I.; Turri, A.; Xia, W.; Norlindh, B.; Johansson, A.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration using resorbable membrane and different bone substitutes: Early histological and molecular events. Acta Biomater. 2016, 29, 409–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberius, P.; Dahlin, C.; Linde, A. Role of osteopromotion in experimental bone grafting to the skull: A study in adult rats using a membrane technique. Int. J. Oral Maxillofac. Surg. 1992, 50, 829–834. [Google Scholar] [CrossRef]
- Lundgren, D.; Laurell, L.; Gottlow, J.; Rylander, H.; Mathisen, T.; Nyman, S.; Rask, M. The influence of the design of two different bioresorbable barriers on the results of guided tissue regeneration therapy. An intra-individual comparative study in the monkey. J. Periodontol. 1995, 66, 605–612. [Google Scholar] [PubMed]
- Nociti, F.H., Jr.; César, N.J.; Carvalho, M.D.; Sallum, E.A. Bone density around titanium implants may be influenced by intermittent cigarette smoke inhalation: A histometric study in rats. Int. J. Oral Maxillofac. Implants 2002, 17, 347–352. [Google Scholar] [PubMed]
- Warnakulasuriya, S.; Sutherland, G.; Scully, C. Tobacco, oral cancer, and treatment of dependance. Oral Oncol. 2005, 41, 244–260. [Google Scholar] [CrossRef] [PubMed]
- César-Neto, J.B.; Rosa, E.F.; Pannuti, C.M.; Romito, G.A. Smoking and periodontal tissues: A review. Braz. Oral Res. 2012, 26 (Suppl. 1), 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yuhara, S.; Kasagi, S.; Inoue, A.; Otsuka, E.; Hirose, S.; Hagiwara, H. Effects of nicotine on cultured cells suggests that it can influence the formation and resorption of bone. Eur. J. Pharmacol. 1999, 383, 387–393. [Google Scholar] [CrossRef]
- Cesar-Neto, J.B.; Duarte, P.M.; Sallum, E.A.; Barbieri, D.; Moreno, H., Jr.; Nociti Júnior, F.H. A comparative study on the effect of nicotine administration and cigarette smoke inhalation on bone healing around titanium implants. J. Periodontol. 2003, 74, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Gullihorn, L.; Karpman, R.; Lippiello, L. Differential effects of nicotine and smoke condensate on bone cell metabolic activity. J. Orthop. Trauma 2005, 19, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Strietzel, F.P.; Reichard, P.A.; Kale, A.; Kulkarni, M.; Wegner, B.; Kuchler, I. Smoking interferes with the prognosis of dental implant treatment: A sistematic review and meta-analysis. J. Clin. Periodontol. 2007, 34, 523–544. [Google Scholar] [CrossRef]
- Giorgetti, A.P.O.; César-Neto, J.B.; Ruiz, K.G.S.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Cigarette smoke inhalation modulates gene expression in sites of bone healing: A study in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 447–452. [Google Scholar] [CrossRef]
- Campos, J.M.; Prati, A.J.; Cirano, F.R.; Pimentel, S.P.; Pastore, G.P.; Pecorari, V.G.; Ribeiro, F.V.; Casati, M.Z.; Casarin, R.C. Smoking Modulates Gene Expression of Type I Collagen, Bone Sialoprotein, and Osteocalcin in Human Alveolar Bone. Int. J. Oral Maxillofac. Surg. 2015, 73, 2123–2131. [Google Scholar] [CrossRef]
- Baig, M.R.; Rajan, M. Effects of smoking on the outcome of implant treatment: A literature review. Indian J. Dent. Res. 2007, 18, 190–195. [Google Scholar] [CrossRef]
- Carcuac, O.; Jansson, L. Peri-implantitis in a specialist clinic of periodontology. Clinical features and risk indicators. Swed. Dent. J. 2010, 34, 53–61. [Google Scholar]
- Kasat, V.; Ladda, R. Smoking and dental implants. J. Int. Soc. Prev. Community Dent. 2012, 2, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Giampietro, P.F.; McCarty, C.; Mukesh, B.; McKiernan, F.; Wilson, D.; Shuldiner, A.; Liu, J.; LeVasseur, J.; Ivacic, L.; Kitchner, T.; Ghebranious, N. The role of cigarette smoking and statins in the development of postmenopausal osteoporosis: A pilot study utilizing the Marshfield Clinic Personalized Medicine Cohort. Osteoporos. Int. 2010, 21, 467–477. [Google Scholar] [CrossRef]
- Gao, S.G.; Li, K.H.; Xu, M.; Jiang, W.; Shen, H.; Luo, W.; Xu, W.S.; Tian, J.; Lei, G.H. Bone turnover in passive smoking female rat: Relationships to change in bone mineral density. BMC Musculoskelet. Disord. 2011, 12, 131. [Google Scholar] [CrossRef]
- Wong, P.K.K.; Christie, J.J.; Wark, J.D. The effects of smoking on bone health. Clin. Sci. 2007, 113, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Ajiro, Y.; Tokuhashi, Y.; Matsuzaki, H.; Nakajima, S.; Ogawa, T. Impact of passive smoking on the bones of rats. Orthopedics 2010, 33, 1–7. [Google Scholar] [CrossRef]
- Hapidin, H.; Othman, F.; Nirwana, I.; Shuid, A.N.; Luke, D.A.; Mohamed, N. Negative effects of nicotine on bone-resorbing cytokines and bone histomorphometric parameters in the male rat. J. Bone Miner. Metab. 2007, 25, 93–98. [Google Scholar] [CrossRef]
- Law, M.R.; Hackshaw, A.K. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: Recognition of a major effect. BMJ 1997, 315, 840–845. [Google Scholar] [CrossRef]
- Bonfante, S.; Bosco, A.F.; Luize, D.S.; de Almeida, J.M.; Cestari, T.M.; Taga, R. Influence of nicotine on healing process of autogenous bone block grafts in the mandible: A histomorphometric study in rats. Int. J. Oral Maxillofac. Implants 2008, 23, 437–444. [Google Scholar]
- Nascimento, R.D.; Cardoso, P.E.; De Marco, A.C.; de Lima, L.A.; Jardini, M.A.N. Influence of osteopenia in autogenous bone graft healing with or without expanded polytetrafluoroethylene membranes: Histologic and histomorphometric study in rats. Int. J. Oral Maxillofac. Implants 2009, 24, 1074–1082. [Google Scholar]
- Tera, T.M.; Nascimento, R.D.; Prado, R.F.; Santamaria, M.P.; Jardini, M.A.N. Immunolocalization of markers for bone formation during guided bone regeneration in osteopenic rats. J. Appl. Oral Sci. 2014, 22, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Tera, T.M.; Prado, R.F.; De Marco, A.C.; Santamaria, M.P.; Jardini, M.A.N. The RANK/RANKL/OPG interaction in the repair of autogenous bone grafts in female rats with estrogen deficiency. Braz. Oral Res. 2014, 28, 1–9. [Google Scholar] [CrossRef]
- Matsuzaka, K.; Shimono, M.; Inoue, T. Characteristics of newly formed bone during guided bone regeneration: Observations by laser scanning microscopy. Bull. Tokyo Dent. Coll. 2001, 42, 225–234. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials—Biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 106–116. [Google Scholar] [CrossRef]
- Hämmerle, C.H.; Schmid, J.; Olah, A.J.; Lang, N.P. A novel model system for the study of experimental guided bone formation in humans. Clin. Oral Implants Res. 1996, 7, 38–47. [Google Scholar] [CrossRef]
- Duarte, P.M.; César Neto, J.B.; Gonçalves, P.F.; Sallum, E.A.; Nociti, J.F. Estrogen deficiency affects bone healing around titanium implants: A histometric study in rats. Implant Dent. 2003, 12, 340–346. [Google Scholar] [CrossRef]
- Mardas, N.; Kostopoulos, L.; Karring, T. Bone and suture regeneration in calvarial defects by e-PTFE-membranes and demineralized bone matrix and the impact on calvarial growth: An experimental study in the rat. J. Craniofac. Surg. 2002, 13, 453–462. [Google Scholar] [CrossRef]
- Calciolari, E.; Mardas, N.; Dereka, X.; Kostomitsopoulos, N.; Petrie, A.; Donos, N. The effect of experimental osteoporosis on bone regeneration: Part 1, histology findings. Clin. Oral Implants Res. 2017, 28, e101–e110. [Google Scholar] [CrossRef]
- Retzepi, M.; Calciolari, E.; Wall, I.; Lewis, M.P.; Donos, N. The effect of experimental diabetes and glycaemic control on guided bone regeneration: Histology and gene expression analyses. Clin. Oral Implants Res. 2017, 29, 139–154. [Google Scholar] [CrossRef]
- Gielkens, P.F.; Schortinghuis, J.; de Jong, J.R.; Paans, A.M.; Ruben, J.L.; Raghoebar, G.M.; Stegenga, B.; Bos, R.R. The influence of barrier membranes on autologous bone grafts. J. Dent. Res. 2008, 87, 1048–1052. [Google Scholar] [CrossRef]
- Luvizuto, E.R.; Dias, S.M.; Queiroz, T.P.; Okamoto, T.; Garcia, I.R., Jr.; Okamoto, R.; Dornelles, R.C. Osteocalcin immunolabeling during the alveolar healing process in ovariectomized rats treated with estrogen or raloxifene. Bone 2010, 46, 1021–1029. [Google Scholar] [CrossRef]
- Luvizuto, E.R.; Queiroz, T.P.; Dias, S.M.; Okamoto, T.; Dornelles, R.C.; Garcia, I.R., Jr.; Okamoto, R. Histomorphometric analysis and immunolocalization of RANKL and OPG during the alveolar healing process in female ovariectomized rats treated with oestrogen or raloxifene. Arch. Oral Biol. 2010, 55, 52–59. [Google Scholar] [CrossRef]
- Carvalho, M.D.; Benatti, B.B.; César-Neto, J.B.; Nociti, F.H., Jr.; da Rocha Nogueira Filho, G.; Casati, M.Z.; Sallum, E.A. Effect of cigarette smoke inhalation and estrogen deficiency on bone healing around titanium implants: A histometric study in rats. J. Periodontol. 2006, 77, 599–605. [Google Scholar] [CrossRef]
- Saldanha, J.B.; Pimentel, S.P.; Casati, M.Z.; Sallum, E.A.; Barbieri, D.; Moreno, H., Jr.; Nociti, F.H., Jr. Guided bone regeneration may be negatively influenced by nicotine administration: A histologic study in dogs. J. Periodontol. 2004, 75, 565–571. [Google Scholar] [CrossRef]
- Machado, G.J.; Dias, S.M.; Bosco, A.F.; Okamoto, T.; Bedran de Castro, J.C.; Dornelles, R.C. Action of nicotine and ovariectomy on bone regeneration after tooth extraction in rats. Int. J. Oral Maxillofac. Surg. 2010, 68, 2675–2681. [Google Scholar] [CrossRef]
- Mezquita-Raya, P.; de la Higuera, M.; García, D.F.; Alonso, G.; Ruiz-Requena, M.E.; de Dios Luna, J.; Escobar-Jiménez, F.; Muñoz-Torres, M. The contribution of sérum osteoprotegerin to bone mass and vertebral fractures in postmenopausal women. Osteoporos. Int. 2005, 16, 1368–1374. [Google Scholar] [CrossRef]
- Weiss, R.J.; Erlansson Harris, H.; Wick, M.C.; Wretenberg, P.; Stark, A.; Palmblad, K. Morphological characterizationof receptor activator of NFkappaB ligant (RANKL) and IL-1beta expression in rodent collagen-induced arthritis. Scand. J. Immunol. 2005, 62, 55–62. [Google Scholar] [CrossRef]
- Yoon, V.; Maalouf, N.M.; Sakhaee, K. The effects of smoking on bone metabolism. Osteoporos. Int. 2012, 23, 2081–2092. [Google Scholar] [CrossRef]
- Ojima, M.; Hanioka, T. Destructive effects of smoking on molecular and genetic factors of periodontal disease. Tob. Induc. Dis. 2010, 8, 4. [Google Scholar] [CrossRef]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Saito, C.T.; Gulinelli, J.L.; Panzarini, S.R.; Garcia, V.G.; Okamoto, R.; Okamoto, T.; Sonoda, C.K.; Poi, W.R. Effect of low-level laser therapy on the healing process after tooth replantation: A histomorphometrical and immunohistochemical analysis. Dent. Traumatol. 2011, 27, 30–39. [Google Scholar] [CrossRef]
- Ivanovski, S.; Li, H.; Daley, T.; Bartold, P.M. An immunohistochemical study of matrix molecules associated with barrier membrane-mediated periodontal wound healing. J. Periodontal Res. 2000, 35, 115–126. [Google Scholar] [CrossRef]
- Hawthorne, A.C.; Xavier, S.P.; Okamoto, R.; Salvador, S.L.; Antunes, A.A.; Salata, L.A. Immunohistochemical, tomographic, and histological study on onlay bone graft remodeling. Part III: Allografts. Clin. Oral Implants Res. 2013, 24, 1164–1172. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretto Nunes, C.M.; Bernardo, D.V.; Ferreira, C.L.; Gomes, M.F.; De Marco, A.C.; Santamaria, M.P.; Jardini, M.A.N. Influence of Cigarette Smoke Inhalation on an Autogenous Onlay Bone Graft Area in Rats with Estrogen Deficiency: A Histomorphometric and Immunohistochemistry Study. Int. J. Mol. Sci. 2019, 20, 1854. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20081854
Moretto Nunes CM, Bernardo DV, Ferreira CL, Gomes MF, De Marco AC, Santamaria MP, Jardini MAN. Influence of Cigarette Smoke Inhalation on an Autogenous Onlay Bone Graft Area in Rats with Estrogen Deficiency: A Histomorphometric and Immunohistochemistry Study. International Journal of Molecular Sciences. 2019; 20(8):1854. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20081854
Chicago/Turabian StyleMoretto Nunes, Camilla Magnoni, Daniella Vicensotto Bernardo, Camila Lopes Ferreira, Mônica Fernandes Gomes, Andrea Carvalho De Marco, Mauro Pedrine Santamaria, and Maria Aparecida Neves Jardini. 2019. "Influence of Cigarette Smoke Inhalation on an Autogenous Onlay Bone Graft Area in Rats with Estrogen Deficiency: A Histomorphometric and Immunohistochemistry Study" International Journal of Molecular Sciences 20, no. 8: 1854. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20081854