Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues
Abstract
:1. Introduction
2. Repertoire of Membrane Lipids
- Energy storage: lipid droplets used for this function contain mainly triacylglycerol and steryl esters thanks to their relatively reduced state. These anhydrous reservoirs are needed for the efficient storage of caloric reserves and as stores of fatty acid and sterol components for membrane biogenesis.
- Compartmentalization: the milieu of cellular membranes is made of lipids of amphipathic nature, comprising both a hydrophobic and a hydrophilic portion. This amphipathic nature provides the physical basis for spontaneous membrane formation because the hydrophobic moieties are prone to self-associate when dissolved in water. This predisposition to self-associate enabled the segregation of an internal milieu from the external milieu when the first cells originated. Later on, this scheme was repeated inside the cell to generate discrete organelles allowing, first, the separation of specific chemical reactions, second, the limitation in the spreading of reaction products and, third, an improvement in biochemical efficiency. Furthermore, lipids are responsible of membrane ability of budding, tubulation, fission and fusion, all them indispensable for cell division, biological reproduction and intracellular membrane trafficking.
- Signaling: in signal transduction, lipids first define membrane domains that allow the aggregation and dispersion of particular proteins, and subsequently organize secondary signaling or effector complexes; they can also act as first and second messengers. The rupture of amphipathic lipids generates bipartite signaling elements, which can be spread both within a membrane (by hydrophobic portions of the molecule) and through the cytosol (by soluble/polar portions of the molecule).
3. Lipids in Sustaining Organelle Structure, Function and Identity
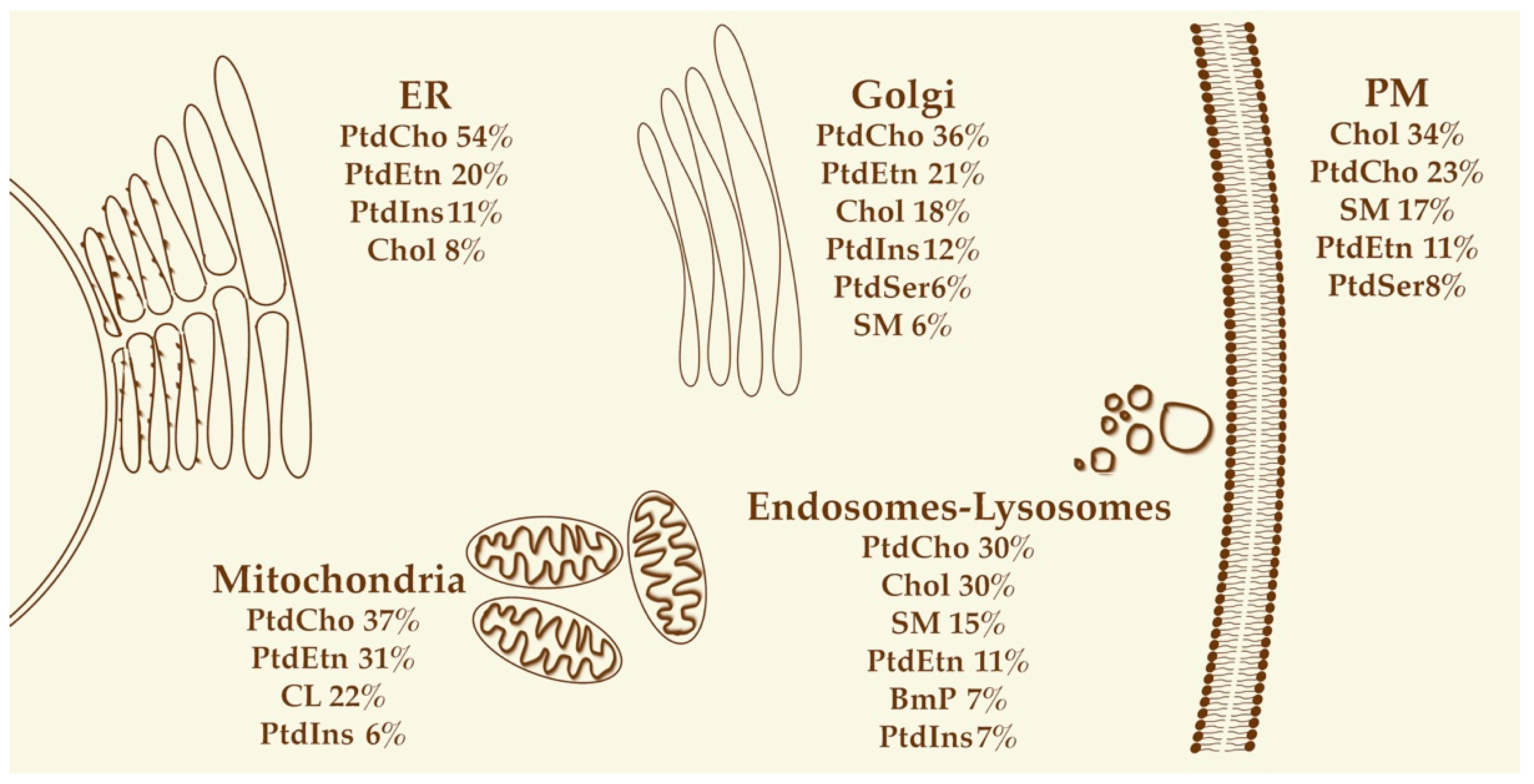
3.1. Endoplasmic Reticulum
3.2. Golgi
3.3. Plasma Membrane
Endosomes
3.4. Mitochondria
3.5. Lysosomes
3.6. Nuclear Membrane
3.6.1. Nuclear Size
3.6.2. Nuclear Phospholipid Regulation of Chromatin
4. Cellular Mechanisms of Physicochemical Membrane Homeostasis
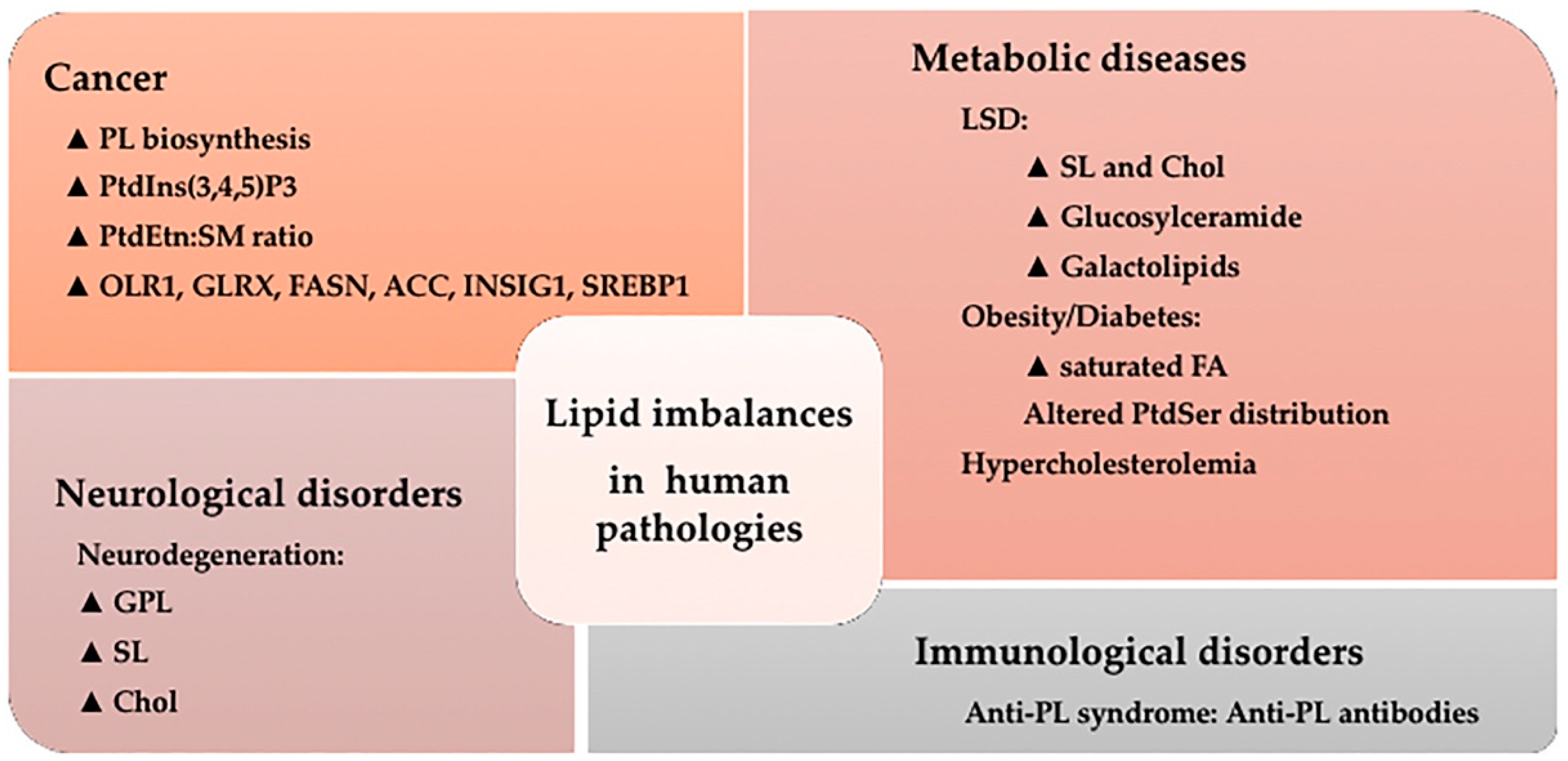
5. Lipid Imbalances and Human Pathologies
5.1. Cancer
- Total SM levels are decreased and PtdEtn levels are increased in tumor cells compared to non-tumor controls [138]. As a consequence, the PtdEtn:SM ratio in tumor cells is ca. 10-fold the ratio in normal cells. In fact, PtdEtn:SM ratio might work as a switch where high PtdEtn:SM ratio is a ‘ON’ state allowing propagation of proliferation signals received at the PM, while a low PtdEtn:SM ratio is a ‘OFF’ state where the PM is impaired for transduction of proliferative signals [139].
- Other SL involved in cancer pathology include Cer, Sph and S1P (reviewed in [140]). Cer mediates numerous cell-stress responses, such as induction of apoptosis [141] and cell senescence [142], whereas S1P in contrast exerts its role in cell survival, migration, and inflammation [143]. Further investigations point to an abnormal SL signaling in carcinogenesis of various types of cancer due to alterations in the activity of enzymes that participate in the metabolism of SL [144].
- Other lipid metabolism genes related with cancer are Oxidized Low Density Lipoprotein Receptor 1 (OLR1) and Glutaredoxin (GLRX), which are upregulated in breast and prostate cancer tissues [145]. The oncogenic antigen-519, a molecular marker found in breast cancer patients with poor prognosis, was identified as FA Synthase (FASN) already twenty-five years ago [146]. Other proteins related to FA biosynthesis and lipid metabolism regulation, such as acetyl-CoA carboxylase (ACC), Insulin induced gene 1 (INSIG1), and sterol regulatory element-binding protein 1 (SREBP1), are highly expressed in breast cancer tumors and associated with low patient survival [147] while colorectal carcinoma risk has been associated with hepatic lipase polymorphisms [148]. Alterations in lysosomal SL metabolism are another trait of many cancers [149].
- ▪
- Disruption of normal tissue architecture: The collapse of normal tissue architecture is a mark of malignancy. Polarized epithelia turn into disorganized structures that can occupy the adjacent tissues. In epithelial tissues, aberrant FA synthesis was linked to the loss of cell polarity. Also, the ordinary expression of SREBPs is needed to maintain the apical surface of normal epithelial cells and is lost in many cancers (reviewed in [135])
- ▪
- Cancer cell migration: Cell migration stimulation by pro-migratory signaling lipids, such as DAG, LPA and prostaglandins, has been well characterized (reviewed in [150]). In addition, other findings suggest that dietary-derived lipids might enlarge the overall lipid composition of malignant cells and so influence multiple signaling events within tumors [151].
- ▪
- Interaction of cancer cells with components of the tumor stroma: This is another key element affecting tumor growth [152] and lipids were also involved in this communication. Examples include the support to cancer-associated fibroblasts by the expression of FASN [153], the compromise of proper macrophage functioning upon FA biosynthesis [134,154] and of proper immune response upon prostaglandin presence [155].
- ▪
- Lipid metabolic reprogramming in cancer cells: cancer cells display an enlarged metabolic inventory that allows the flexibility to survive and grow in the severe tumor environment. Highly proliferative cancer cells display a strong lipid and Chol avidity, which they fulfill by raising the incorporation of exogenous (or dietary) lipids or increasing their endogenous synthesis. Excessive lipids and cholesterol in cancer cells stored in lipid droplets are considered marks of cancer aggressiveness (reviewed in [156]).
5.2. Metabolic Diseases
- ▪
- In the first example, excessive intake of saturated FA is highly toxic for hepatocytes due to its limited capacity to integrate them into TG [163]. In parallel, an elevated degree of membrane saturation disrupts calcium homeostasis and triggers liver ER stress [164]. Chronic activation of ER stress dysregulates lipid homeostasis and might lead to dyslipidemia, insulin resistance, type II diabetes, and obesity [163].
- ▪
- A second example involves the previously introduced TFEB, transcription factor partially dictating lysosome biogenesis. As above mentioned, TFEB participates in autophagy and in the clearance of lipid droplets. Furthermore, it governs liver lipid catabolism and energy metabolism interfering with peroxisome proliferator-activated receptor-γ (PPAR-γ) signaling [165]. In obese animals, TFEB overexpression rescues obesity and associated metabolic syndrome by promoting lipophagy [166].
5.3. Neurological Disorders
5.4. Immunological Disorders
5.5. Regulation of Cellular Activities by Prenylation
6. New Lipid-Based Therapeutic Avenues: Membrane Lipid Therapy
6.1. Molecular Bases of Targeting the Plasma Membrane
- Direct regulation through membrane structure modification: Dietary lipids and environmental changes modify cell membranes changing their properties and microdomain organization, thus controlling the localization and activity of proteins such as G proteins (interaction with membrane and downstream signaling) [199,200], the transcription of proteins involved in stress response such as heat shock proteins (Hsp) [201], or the production of second messengers such as Cer [202]. Thus, MUFA treatments can change the order of Lo and Ld microdomains.
- Regulation of enzymatic activity to alter membrane lipid levels: Enzymatic activity of SMS and other enzymes from SL metabolism are altered in cancer and consequently modify membrane composition and structure. Hydroxyl-C18 unsaturated fatty acids (hydroxyoleic, hydroxilinoleic, hydroxy-α-linolenic and hydroxy-γ-linolenic acids) have effects on SMS activity [138].
- Modification of gene expression that results in alterations of membrane lipid composition: this gene expression change might affect the activity of an enzyme, protein-lipid interactions or protein-protein interaction. DNA-associated PL are found in nuclear membrane [203] and their effects on nuclear functions have been documented [103]. Evidence of this type of MLT include the increased SM levels in differentiated cells in contrast to low levels of this lipid on proliferating cells [138]. This increase does not take place with SM addition but for SMS increased activity [138,139], which supports the concept of MLT-induced gene expression alteration.
- Lipid alterations that affect protein-protein interactions in specific membrane microdomains: The alteration of lipid ratios or the presence of particular lipids in membranes cause changes in protein-protein interactions. For example, low PtdEtn/SM ratios reduce Ras interactions both with membrane components and with downstream partners, in turn, inhibiting the transduction through proliferative signaling cascades and preventing proliferation in cancer cells [139].
- Direct MLT-drug binding to a protein that alters its membrane binding affinity or that of other signaling proteins: In that case, the MLT formulated molecule binds to a protein rather than a lipid. This is the case of prenylation inhibitors, which prevent Ras from binding to membrane and subsequently inhibit cancer cell proliferation while inducing cell differentiation and cell death [139].
6.2. Development of Membrane Lipid Therapy in Different Therapeutic Areas
- Oncology. The basis of developing MLT in oncology comes from the finding that membrane lipid composition might work as a switch allowing or compromising propagation of proliferation signals received at the PM in tumor cells [196,207]. Many molecules have been developed since the discovery membrane-altering mechanism of action of doxorubicin [199]. One of the most promising molecules is rationally-designed 2-OHOA (2-hydroxyoleic acid) [208], which is currently being tested in clinical trials for the treatment of glioma. 2-OHOA activates SMS increasing its product, SM, and decreasing its substrate PtdEtn in membranes of cancer cells but not of healthy cells because of the higher levels of SM found in tumor cells [138]. 2OHOA was the first rationally designed MLT molecule to arrive clinical trials. It has shown good pharmaceutical efficacy and safety against cancer in humans (ClinicalTrials.gov identifier #NCT01792310). After a first-in-man phase I/IIA trial in patients with solid tumors, 43% glioma patients responded to treatment, although this percentage almost doubled (ca. 80%) if patients previously pretreated with avastin were disregarded [6]. Other molecules being explored in oncology follow here: 2-hydroxylinoleic acid is on phase II clinical trial. This PUFA binds to membrane and inhibits the Akt/mTORC1 axis and induces specific cancer cell autophagy [209]. Hydroxytriolein is a triacylglycerol mimetic synthetic lipid analogue of triolein shown to block cancer cell growth in vitro through the β-catenin pathway, downregulation of the MEK-ERK axis, and production of Reactive Oxygen Species and apoptosis [210]. Worth mentioning are propofol-docosahexaenoic acid (P-DHA) and its analogue edelfosine (reviewed in [6]). Examples of anticancer therapies based on inhibitors targeting enzyme regulators of lipid metabolism are orlistat (Roche Xenical®), a FASN inhibitor administered in the treatment of breast cancer [211]; and ABC294640, a sphingosine kinase 2 and dihydroceramide desaturase inhibitor currently under an Ib/II clinical trial (ClinicalTrials.gov identifier #NCT02757326).
- Metabolic and cardiovascular diseases: The use of dietary lipids for the treatment of diabetes or obesity is a clear case of MLT. For example, body weight reductions where achieved in rats with daily supplements of olive oil (made of 70%–80% oleic acid) but not with supplements of its trans analogue elaidic acid, due to the different structure of both FA [44,212]. Furthermore, oleic acid analogues induce reduction of body weight in rats by promoting overexpression of uncoupling proteins UCP1 and UCP3 and decreasing food intake [212]. A high intake of oleic acid has also been shown to improve glycemic status and reduce saturated FA levels of diabetic patients while increasing those of unsaturated FA [159]. In the case of cardiovascular conditions, high oleic acid intake and ω-3 FA consumption were linked to reduced blood pressure values [213,214]. This reduction is even greater with 2-hydroxioleic acid treatment [215,216]. Furthermore, unsaturated FA are cardioprotective [217].
- Neurodegenerative disorders: Behind adipose tissue, the central nervous system concentrates the largest depot of lipids in the body making it a primary target for MLT strategies. Alzheimer’s disease risk has been inversely associated with ω-3 polyunsaturated fatty acid (PUFA) consumption [218]. In detail, the altered amyloid precursor protein (APP) proteolysis upon DHA abundance was the basis to design 2-hydroxioleic-DHA (2-OHDHA). A four-month treatment with 2-OHDHA in a severe Alzheimer’s disease mice model (5XFAD) restored cognition to control values [219,220]. Spinal cord injury might also benefit from MLT approaches (reviewed in [19]). Albumin-oleic acid complex induces significant motor recovery (~ 40%) in rats with spinal cord injury (SCI) [221], ameliorating both spasticity and pain. The oleic acid analogue NFX88 (Neurofix) is undergoing clinical trials for the treatment of neuropathic pain in patients with SCI. Finally, a phase II/III clinical trial (ClinicalTrials.gov identifier #NCT00706147) is being developed to evaluate the efficacy of the lipid interacting hydroxylamine derivative arimoclomol in familial amyotrophic lateral sclerosis.
- Other conditions that are bound to be ameliorated using MLT include infectious diseases, chemotherapeutic neuropathy, wound healing, retinopathies, nephropathies, acetaminophen liver toxicity, sunburn, ischemia reperfusion, intracranial hemorrhage, atrial fibrillation, vascular hypertension damage, and myocardial infarction as reviewed in [19].
7. Future Directions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-OHDHA | 2-hydroxydocosahexanoic acid |
| 2-OHOA | 2-hydroxyoleic acid |
| ACC | acetyl-CoA carboxylase |
| ALPS | amphipathic lipid packing sensor |
| APP | amyloid precursor protein |
| APS | anti-phospholipid syndrome |
| BmP | bis(monoacylglycero)phosphate |
| C1P | ceramide-1-phosphate |
| CLIC | clathrin-independent carrier |
| CDP-DAG | cytidine diphosphate diacylglycerol |
| Cer | ceramide |
| CerK | ceramide kinase |
| Chol | cholesterol |
| CL | cardiolipin |
| COP | coat protein complex |
| DAG | diacylglycerol |
| DMC | DNA–membrane complexes |
| ER | endoplasmic reticulum |
| ESCRT | endosomal sorting complexes required for transport |
| FASN | fatty acid synthase |
| FD | Fabry disease |
| FTase | farnesyltransferase |
| FTI | farnesyltransferase inhibitor |
| GalCer | galactosylceramide |
| GD | Gaucher disease |
| GEEC | (GptdIns)-anchor-enriched endocytic compartment |
| GEF | guanine nucleotide exchange factors |
| GGTase | geranylgeranyltransferase |
| GGTI | geranylgeranyltransferase inhibitor |
| GlcCer | glucosylceramide |
| GLRX | glutaredoxin |
| GPL | glycerophospholipids |
| GptdIns | glycosylphosphatidylinositol |
| GSL | glycosphingolipid |
| GTPase | guanosine-5′-triphosphate hydrolase |
| Hsp | heat shock protein |
| IMM | inner mitochondrial membrane |
| IMS | intermembrane space |
| INM | inner nuclear membrane |
| INSIG1 | insulin induced gene 1 |
| KD | Krabbe disease |
| LA | lupus anticoagulant |
| LacCer | lactosylceramide |
| Ld | Liquid-disordered |
| LDL | low-density lipoprotein |
| LECA | last eukaryotic common ancestor |
| Lo | Liquid-ordered |
| LPA | lysoPA |
| LPC | lysoPtdCho |
| LSD | lysosomal storage disease |
| LTP | lipid transfer protein |
| LUCA | last universal common ancestor |
| MAM | mitochondria associated membrane |
| MCS | membrane contact site |
| MLT | membrane lipid therapy |
| MUFA | monounsaturated fatty acid |
| NE | nuclear envelope |
| NM | nuclear membrane |
| NPC | nuclear pore complex |
| NSF | N-ethylmaleimide-sensitive factor |
| OLR1 | oxidized low density lipoprotein receptor 1 |
| OMM | outer mitochondrial membrane |
| ONM | outer nuclear membrane |
| P4-ATPase | type 4 P-type ATPases |
| P-DHA | propofol-docosahexaenoic acid |
| PA | phosphatidic acid |
| PKC | protein kinase C |
| PL | phospholipids |
| PM | plasma membrane |
| PPAR-γ | peroxisome proliferator-activated receptor-γ |
| PPIn | polyphosphoinositides |
| PtdCho | phosphatidylcholine |
| PtdEtn | phosphatidylethanolamine |
| PtdGro | phosphatidylglycerol |
| PtdIns | phosphatidylinositol |
| PtdIns(1,4,5)P3 | phosphatidylinositol 1,4,5- trisphosphate |
| PtdIns(3,4,5)P3 | phosphatidylinositol 3,4,5-trisphosphate |
| PtdIns(4,5)P2 | phosphatidylinositol 4,5- bisphosphate |
| PtdIns4P | phosphatidylinositol 4-phosphate |
| PtdInsP | phosphatidylinositol phosphate |
| PtdSer | phosphatidylserine |
| SL | sphingolipids |
| SM | sphingomyelin |
| SMase | sphingomyelinase |
| SMS | sphingomyelin synthase |
| SNARE | soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| SPC | sphingosylphosphorylcholine |
| Sph | sphingosine |
| SphK | sphingosine kinase |
| SREBP | sterol regulatory element-binding protein |
| TFEB | transcription factor EB |
| TMDs | transmembrane domains |
| UCP | uncoupling protein |
References
- Gould, S.B. Membranes and evolution. Curr. Biol. 2018, 28, R381–R385. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.; Evans, E.; Mouritsen, O.G. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q. Rev. Biophys. 1991, 24, 293. [Google Scholar] [CrossRef]
- Bloom, M.; Mouritsen, O.G. The Evolution of Membranes. In Handbook of Biological Physics; Elsevier BV: Amsterdam, The Netherlands, 1995; Vol. 1, pp. 65–95. ISBN 0008-4042. [Google Scholar]
- Karnovsky, M.J. The concept of lipid domains in membranes. J. Cell Biol. 1982, 94, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribá, P.V. Membrane-lipid therapy: A historical perspective of membrane-targeted therapies — From lipid bilayer structure to the pathophysiological regulation of cells. Biochim. Biophys. Acta BBA - Biomembr. 2017, 1859, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E. Molecular and global structure and dynamics of membranes and lipid bilayers. Can. J. Phys. 1990, 68, 999–1012. [Google Scholar] [CrossRef]
- Dacks, J.B.; Field, M.C. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J. Cell Sci. 2007, 120, 2977–2985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deamer, D.W. Boundary structures are formed by organic components of the Murchison carbonaceous chondrite. Nature 1985, 317, 792–794. [Google Scholar] [CrossRef]
- Morowitz, H.J.; Heinz, B.; Deamer, D.W. The chemical logic of a minimum protocell. Orig. Life Evol. Biosph. 1988, 18, 281–287. [Google Scholar] [CrossRef]
- Glöckner, F.O.; Kube, M.; Bauer, M.; Teeling, H.; Lombardot, T.; Ludwig, W.; Gade, D.; Beck, A.; Borzym, K.; Heitmann, K.; et al. Complete genome sequence of the marine planctomycete Pirellula sp. strain 1. Proc. Natl. Acad. Sci. USA 2003, 100, 8298–8303. [Google Scholar] [CrossRef] [Green Version]
- Ranea, J.A.G.; Sillero, A.; Thornton, J.M.; Orengo, C.A. Protein Superfamily Evolution and the Last Universal Common Ancestor (LUCA). J. Mol. Evol. 2006, 63, 513–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifacino, J.S.; Glick, B.S. The Mechanisms of Vesicle Budding and Fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, A.; Simons, K. Lipidomics: coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef]
- Ernst, R.; Ballweg, S.; Levental, I. Cellular mechanisms of physicochemical membrane homeostasis. Curr. Opin. Cell Biol. 2018, 53, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [Green Version]
- Holthuis, J.C.M.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef]
- De Kroon, A.I.P.M.; Rijken, P.J.; De Smet, C.H. Checks and balances in membrane phospholipid class and acyl chain homeostasis, the yeast perspective. Prog. Lipid Res. 2013, 52, 374–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.; Balogh, G.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog. Lipid Res. 2015, 59, 38–53. [Google Scholar] [CrossRef] [Green Version]
- Van Meer, G.; Lisman, Q. Sphingolipid Transport: Rafts and Translocators. J. Biol. Chem. 2002, 277, 25855–25858. [Google Scholar] [CrossRef] [Green Version]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Hullin-Matsuda, F.; Taguchi, T.; Greimel, P.; Kobayashi, T. Lipid compartmentalization in the endosome system. Semin. Cell Dev. Biol. 2014, 31, 48–56. [Google Scholar] [CrossRef]
- Diaz-Rohrer, B.B.; Levental, K.R.; Simons, K.; Levental, I. Membrane raft association is a determinant of plasma membrane localization. Proc. Natl. Acad. Sci. USA 2014, 111, 8500–8505. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Rohrer, B.; Levental, K.R.; Levental, I. Rafting through traffic: Membrane domains in cellular logistics. Biochim. Biophys. Acta BBA - Biomembr. 2014, 1838, 3003–3013. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, H.J.; Stevens, T.J.; Munro, S. A Comprehensive Comparison of Transmembrane Domains Reveals Organelle-Specific Properties. Cell 2010, 142, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Lorent, J.H.; Diaz-Rohrer, B.; Lin, X.; Spring, K.; Gorfe, A.A.; Levental, K.R.; Levental, I. Structural determinants and functional consequences of protein affinity for membrane rafts. Nat. Commun. 2017, 8, 1219. [Google Scholar] [CrossRef] [Green Version]
- Covino, R.; Ballweg, S.; Stordeur, C.; Michaelis, J.B.; Puth, K.; Wernig, F.; Bahrami, A.; Ernst, A.M.; Hummer, G.; Ernst, R. A Eukaryotic Sensor for Membrane Lipid Saturation. Mol. Cell 2016, 63, 49–59. [Google Scholar] [CrossRef]
- Ballweg, S.; Ernst, R. Control of membrane fluidity: the OLE pathway in focus. Biol. Chem. 2017, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loewen, C.J.R. Phospholipid Metabolism Regulated by a Transcription Factor Sensing Phosphatidic Acid. Science 2004, 304, 1644–1647. [Google Scholar] [CrossRef]
- Halbleib, K.; Pesek, K.; Covino, R.; Hofbauer, H.F.; Wunnicke, D.; Hänelt, I.; Hummer, G.; Ernst, R. Activation of the Unfolded Protein Response by Lipid Bilayer Stress. Mol. Cell 2017, 67, 673–684.e8. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.M.; Ballas, L.M.; Coleman, R.A. Lipid topogenesis. J. Lipid Res. 1981, 22, 391–403. [Google Scholar] [PubMed]
- Sprong, H.; Kruithof, B.; Leijendekker, R.; Slot, J.W.; van Meer, G.; van der Sluijs, P. UDP-Galactose:Ceramide Galactosyltransferase Is a Class I Integral Membrane Protein of the Endoplasmic Reticulum. J. Biol. Chem. 1998, 273, 25880–25888. [Google Scholar] [CrossRef] [Green Version]
- Futerman, A.H.; Riezman, H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005, 15, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Holthuis, J.C.M.; Pomorski, T.; Raggers, R.J.; Sprong, H.; Van Meer, G. The Organizing Potential of Sphingolipids in Intracellular Membrane Transport. Physiol. Rev. 2001, 81, 1689–1723. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Goldstein, J.L.; McDonald, J.G.; Brown, M.S. Switch-like Control of SREBP-2 Transport Triggered by Small Changes in ER Cholesterol: A Delicate Balance. Cell Metab. 2008, 8, 512–521. [Google Scholar] [CrossRef]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef]
- Munro, S. An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J. 1995, 14, 4695–4704. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, R.; Trenchi, A.; Gonzalez Montoro, A.; Valdez Taubas, J.; Maccioni, H.J.F. Short transmembrane domains with high-volume exoplasmic halves determine retention of Type II membrane proteins in the Golgi complex. J. Cell Sci. 2013, 126, 5344–5349. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, H.-J.; Orlowski, A.; Rog, T.; Nyholm, T.K.M.; Chai, W.; Feizi, T.; Lingwood, D.; Vattulainen, I.; Simons, K. Lateral sorting in model membranes by cholesterol-mediated hydrophobic matching. Proc. Natl. Acad. Sci. USA 2011, 108, 16628–16633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhaes, M.A.O.; Glogauer, M. Pivotal Advance: Phospholipids determine net membrane surface charge resulting in differential localization of active Rac1 and Rac2. J. Leukoc. Biol. 2010, 87, 545–555. [Google Scholar] [CrossRef]
- Grinstein, S. Imaging signal transduction during phagocytosis: phospholipids, surface charge, and electrostatic interactions. Am. J. Physiol.-Cell Physiol. 2010, 299, C876–C881. [Google Scholar] [CrossRef]
- Bigay, J.; Antonny, B. Curvature, Lipid Packing, and Electrostatics of Membrane Organelles: Defining Cellular Territories in Determining Specificity. Dev. Cell 2012, 23, 886–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanni, S.; Vamparys, L.; Gautier, R.; Drin, G.; Etchebest, C.; Fuchs, P.F.J.; Antonny, B. Amphipathic Lipid Packing Sensor Motifs: Probing Bilayer Defects with Hydrophobic Residues. Biophys. J. 2013, 104, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funari, S.S.; Barceló, F.; Escribá, P.V. Effects of oleic acid and its congeners, elaidic and stearic acids, on the structural properties of phosphatidylethanolamine membranes. J. Lipid Res. 2003, 44, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Lindner, R.; Naim, H.Y. Domains in biological membranes. Exp. Cell Res. 2009, 315, 2871–2878. [Google Scholar] [CrossRef]
- Ibarguren, M.; López, D.J.; Encinar, J.A.; González-Ros, J.M.; Busquets, X.; Escribá, P.V. Partitioning of liquid-ordered/liquid-disordered membrane microdomains induced by the fluidifying effect of 2-hydroxylated fatty acid derivatives. Biochim. Biophys. Acta BBA - Biomembr. 2013, 1828, 2553–2563. [Google Scholar] [CrossRef] [Green Version]
- Epand, R.M. Proteins and cholesterol-rich domains. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1576–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabrey, S.; Mateo, P.L.; Sturtevant, J.M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry 1978, 17, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Menon, A.K. Transbilayer lipid asymmetry. Curr. Biol. 2018, 28, R386–R391. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, T.; Menon, A.K. Lipid flippases and their biological functions. Cell. Mol. Life Sci. 2006, 63, 2908–2921. [Google Scholar] [CrossRef]
- Daleke, D.L. Phospholipid Flippases. J. Biol. Chem. 2007, 282, 821–825. [Google Scholar] [CrossRef]
- Sebastian, T.T.; Baldridge, R.D.; Xu, P.; Graham, T.R. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim. Biophys. Acta 2012, 1821, 1068–1077. [Google Scholar] [CrossRef]
- Bevers, E.M.; Williamson, P.L. Getting to the Outer Leaflet: Physiology of Phosphatidylserine Exposure at the Plasma Membrane. Physiol. Rev. 2016, 96, 605–645. [Google Scholar] [CrossRef] [Green Version]
- van Kruchten, R.; Mattheij, N.J.A.; Saunders, C.; Feijge, M.A.H.; Swieringa, F.; Wolfs, J.L.N.; Collins, P.W.; Heemskerk, J.W.M.; Bevers, E.M. Both TMEM16F-dependent and TMEM16F-independent pathways contribute to phosphatidylserine exposure in platelet apoptosis and platelet activation. Blood 2013, 121, 1850–1857. [Google Scholar] [CrossRef] [Green Version]
- Hochreiter-Hufford, A.E.; Lee, C.S.; Kinchen, J.M.; Sokolowski, J.D.; Arandjelovic, S.; Call, J.A.; Klibanov, A.L.; Yan, Z.; Mandell, J.W.; Ravichandran, K.S. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 2013, 497, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segawa, K.; Suzuki, J.; Nagata, S. Constitutive exposure of phosphatidylserine on viable cells. Proc. Natl. Acad. Sci. USA 2011, 108, 19246–19251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, T.; Gilbert, G.E.; Shi, J.; Silvius, J.; Kapus, A.; Grinstein, S. Membrane Phosphatidylserine Regulates Surface Charge and Protein Localization. Science 2008, 319, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, J.L.M.; Moreira, M.E.C.; Benjamin, A.; Bonomo, A.C.; Barcinski, M.A. Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J. Immunol. Baltim. Md 1950 2006, 176, 1834–1839. [Google Scholar] [CrossRef]
- Lee, B.-C.; Khelashvili, G.; Falzone, M.; Menon, A.K.; Weinstein, H.; Accardi, A. Gating mechanism of the extracellular entry to the lipid pathway in a TMEM16 scramblase. Nat. Commun. 2018, 9, 3251. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef]
- Tafesse, F.G.; Huitema, K.; Hermansson, M.; van der Poel, S.; van den Dikkenberg, J.; Uphoff, A.; Somerharju, P.; Holthuis, J.C.M. Both Sphingomyelin Synthases SMS1 and SMS2 Are Required for Sphingomyelin Homeostasis and Growth in Human HeLa Cells. J. Biol. Chem. 2007, 282, 17537–17547. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Hailemariam, T.K.; Zhou, H.; Li, Y.; Duckworth, D.C.; Peake, D.A.; Zhang, Y.; Kuo, M.-S.; Cao, G.; Jiang, X.-C. Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 2007, 1771, 1186–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, L.J. Lipid rafts: bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J.; Phair, R.D. Lipids and Cholesterol as Regulators of Traffic in the Endomembrane System. Annu. Rev. Biophys. 2010, 39, 559–578. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, D.; Simons, K. Lipid Rafts As a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Bohdanowicz, M.; Grinstein, S. Role of Phospholipids in Endocytosis, Phagocytosis, and Macropinocytosis. Physiol. Rev. 2013, 93, 69–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dove, S.K.; Cooke, F.T.; Douglas, M.R.; Sayers, L.G.; Parker, P.J.; Michell, R.H. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 1997, 390, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Odorizzi, G.; Babst, M.; Emr, S.D. Fab1p PtdIns(3)P 5-Kinase Function Essential for Protein Sorting in the Multivesicular Body. Cell 1998, 95, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Sbrissa, D.; Ikonomov, O.C.; Shisheva, A. PIKfyve, a Mammalian Ortholog of Yeast Fab1p Lipid Kinase, Synthesizes 5-Phosphoinositides. J. Biol. Chem. 1999, 274, 21589–21597. [Google Scholar] [CrossRef] [PubMed]
- Platta, H.W.; Stenmark, H. Endocytosis and signaling. Curr. Opin. Cell Biol. 2011, 23, 393–403. [Google Scholar] [CrossRef]
- Tatsuta, T.; Scharwey, M.; Langer, T. Mitochondrial lipid trafficking. Trends Cell Biol. 2014, 24, 44–52. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Mannella, C.A.; Buttle, K.; Rath, B.K.; Marko, M. Electron microscopic tomography of rat-liver mitochondria and their interactions with the endoplasmic reticulum. BioFactors 1998, 8, 225–228. [Google Scholar] [CrossRef]
- Daum, G.; Vance, J.E. Import of lipids into mitochondria. Prog. Lipid Res. 1997, 36, 103–130. [Google Scholar] [CrossRef]
- Voelker, D.R. Bridging gaps in phospholipid transport. Trends Biochem. Sci. 2005, 30, 396–404. [Google Scholar] [CrossRef]
- Raturi, A.; Simmen, T. Where the endoplasmic reticulum and the mitochondrion tie the knot: The mitochondria-associated membrane (MAM). Biochim. Biophys. Acta BBA - Mol. Cell Res. 2013, 1833, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum–mitochondria contacts: function of the junction. Nat. Rev. Mol. Cell Biol. 2012, 13, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, C.; Voelker, D.R.; Langer, T. Making heads or tails of phospholipids in mitochondria. J. Cell Biol. 2011, 192, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Voelker, D.R. Genetic analysis of intracellular aminoglycerophospholipid traffic. Biochem. Cell Biol. 2004, 82, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Molecular and Cell Biology of Phosphatidylserine and Phosphatidylethanolamine Metabolism. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier BV: Amsterdam, The Netherlands, 2003; pp. 69–111. ISBN 0-12-540075-6. [Google Scholar]
- Daum, G. Lipids of mitochondria. Biochim. Biophys. Acta 1985, 822, 1–42. [Google Scholar] [CrossRef]
- Strauss, J.F.; Kishida, T.; Christenson, L.K.; Fujimoto, T.; Hiroi, H. START domain proteins and the intracellular trafficking of cholesterol in steroidogenic cells. Mol. Cell. Endocrinol. 2003, 202, 59–65. [Google Scholar] [CrossRef]
- Mitchell, T.W.; Buffenstein, R.; Hulbert, A.J. Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): A comparative study using shotgun lipidomics. Exp. Gerontol. 2007, 42, 1053–1062. [Google Scholar] [CrossRef]
- Albert, C.J.; Anbukumar, D.S.; Monda, J.K.; Eckelkamp, J.T.; Ford, D.A. Myocardial Lipidomics. Developments in myocardial nuclear lipidomics. Front. Biosci. 2007, 12, 2750. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Gross, R.W. Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry 1990, 29, 4992–4996. [Google Scholar] [CrossRef] [PubMed]
- Wallner, S.; Schmitz, G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Mandel, H.; Sharf, R.; Berant, M.; Wanders, R.J.A.; Vreken, P.; Aviram, M. Plasmalogen Phospholipids Are Involved in HDL-Mediated Cholesterol Efflux: Insights from Investigations with Plasmalogen-Deficient Cells. Biochem. Biophys. Res. Commun. 1998, 250, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Munn, N.J.; Arnio, E.; Liu, D.; Zoeller, R.A.; Liscum, L. Deficiency in ethanolamine plasmalogen leads to altered cholesterol transport. J. Lipid Res. 2003, 44, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Périchon, R.; Moser, A.B.; Wallace, W.C.; Cunningham, S.C.; Roth, G.S.; Moser, H.W. Peroxisomal Disease Cell Lines with Cellular Plasmalogen Deficiency Have Impaired Muscarinic Cholinergic Signal Transduction Activity and Amyloid Precursor Protein Secretion. Biochem. Biophys. Res. Commun. 1998, 248, 57–61. [Google Scholar] [CrossRef]
- Napolitano, G.; Ballabio, A. TFEB at a glance. J. Cell Sci. 2016, 129, 2475–2481. [Google Scholar] [CrossRef] [Green Version]
- Medina, D.L.; Fraldi, A.; Bouche, V.; Annunziata, F.; Mansueto, G.; Spampanato, C.; Puri, C.; Pignata, A.; Martina, J.A.; Sardiello, M.; et al. Transcriptional Activation of Lysosomal Exocytosis Promotes Cellular Clearance. Dev. Cell 2011, 21, 421–430. [Google Scholar] [CrossRef]
- Nezich, C.L.; Wang, C.; Fogel, A.I.; Youle, R.J. MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and Atg5. J. Cell Biol. 2015, 210, 435–450. [Google Scholar] [CrossRef] [Green Version]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [Green Version]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.R.A.; Saftig, P. Lysosomal storage disorders – challenges, concepts and avenues for therapy: beyond rare diseases. J. Cell Sci. 2019, 132, jcs221739. [Google Scholar] [CrossRef]
- Thelen, A.M.; Zoncu, R. Emerging Roles for the Lysosome in Lipid Metabolism. Trends Cell Biol. 2017, 27, 833–850. [Google Scholar] [CrossRef]
- Jaishy, B.; Abel, E.D. Lipids, lysosomes, and autophagy. J. Lipid Res. 2016, 57, 1619–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapley, E.C.; Starr, D.A. Connecting the nucleus to the cytoskeleton by SUN–KASH bridges across the nuclear envelope. Curr. Opin. Cell Biol. 2013, 25, 57–62. [Google Scholar] [CrossRef]
- Suntharalingam, M.; Wente, S.R. Peering through the Pore: Nuclear Pore Complex Structure, Assembly, and Function. Dev. Cell 2003, 4, 775–789. [Google Scholar] [CrossRef]
- Cascianelli, G.; Villani, M.; Tosti, M.; Marini, F.; Bartoccini, E.; Viola Magni, M.; Albi, E. Lipid Microdomains in Cell Nucleus. Mol. Biol. Cell 2008, 19, 5289–5295. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, A.A. Lipid mediators in the neural cell nucleus: Their metabolism, signaling, and association with neurological disorders. Neuroscientist 2009, 15, 392–407. [Google Scholar] [CrossRef]
- Garcia-Gil, M.; Albi, E. Nuclear Lipids in the Nervous System: What they do in Health and Disease. Neurochem. Res. 2017, 42, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Wu, G. Thematic Review Series: Sphingolipids. Nuclear sphingolipids: metabolism and signaling. J. Lipid Res. 2008, 49, 1176–1186. [Google Scholar] [CrossRef]
- Takagi, M.; Imamoto, N. Control of Nuclear Size by NPC Proteins. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; pp. 571–591. ISBN 978-1-4899-8031-1. [Google Scholar]
- Kuvichkin, V.V. Lipid-nucleic acids interactions as base for organization and expression of cellular genome. Int. J. Quantum Chem. 2010, 110, 120–126. [Google Scholar] [CrossRef]
- Jacob, F.; Brenner, S.; Cuzin, F. On the Regulation of DNA Replication in Bacteria. Cold Spring Harb. Symp. Quant. Biol. 1963, 28, 329–348. [Google Scholar] [CrossRef]
- Hamann, B.L.; Blind, R.D. Nuclear phosphoinositide regulation of chromatin. J. Cell. Physiol. 2018, 233, 107–123. [Google Scholar] [CrossRef]
- Benoit, G.; Cooney, A.; Giguere, V.; Ingraham, H.; Lazar, M.; Muscat, G.; Perlmann, T.; Renaud, J.-P.; Schwabe, J.; Sladek, F.; et al. International Union of Pharmacology. LXVI. Orphan Nuclear Receptors. Pharmacol. Rev. 2006, 58, 798–836. [Google Scholar] [CrossRef] [Green Version]
- Hazel, J. Thermal Adaptations in Biological Membranes: Is Homeoviscous Adaptation the Explanation? Annu. Rev. Physiol. 1995, 57, 19–42. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta BBA - Rev. Biomembr. 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Marsh, D. Lateral Pressure Profile, Spontaneous Curvature Frustration, and the Incorporation and Conformation of Proteins in Membranes. Biophys. J. 2007, 93, 3884–3899. [Google Scholar] [CrossRef] [Green Version]
- Frolov, V.A.; Shnyrova, A.V.; Zimmerberg, J. Lipid Polymorphisms and Membrane Shape. Cold Spring Harb. Perspect. Biol. 2011, 3, a004747. [Google Scholar] [CrossRef]
- Escribá, P.V. Membrane-lipid therapy: a new approach in molecular medicine. Trends Mol. Med. 2006, 12, 34–43. [Google Scholar] [CrossRef]
- Cockcroft, S.; Raghu, P. Phospholipid transport protein function at organelle contact sites. Curr. Opin. Cell Biol. 2018, 53, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Lange, Y.; Steck, T.L. Active membrane cholesterol as a physiological effector. Chem. Phys. Lipids 2016, 199, 74–93. [Google Scholar] [CrossRef]
- Endapally, S.; Frias, D.; Grzemska, M.; Gay, A.; Tomchick, D.R.; Radhakrishnan, A. Molecular Discrimination between Two Conformations of Sphingomyelin in Plasma Membranes. Cell 2019, 176, 1040–1053.e17. [Google Scholar] [CrossRef]
- Infante, R.E.; Radhakrishnan, A. Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, K.; Ikonen, E. How cells handle cholesterol. Science 2000, 290, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Fairn, G.D.; Schieber, N.L.; Ariotti, N.; Murphy, S.; Kuerschner, L.; Webb, R.I.; Grinstein, S.; Parton, R.G. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J. Cell Biol. 2011, 194, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, P.; Wang, J.; Hua, Z.; Graham, T.R. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc. Natl. Acad. Sci. USA 2004, 101, 10614–10619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alder-Baerens, N.; Lisman, Q.; Luong, L.; Pomorski, T.; Holthuis, J.C.M. Loss of P4 ATPases Drs2p and Dnf3p Disrupts Aminophospholipid Transport and Asymmetry in Yeast Post-Golgi Secretory Vesicles. Mol. Biol. Cell 2006, 17, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Tulodziecka, K.; Diaz-Rohrer, B.B.; Farley, M.M.; Chan, R.B.; Di Paolo, G.; Levental, K.R.; Waxham, M.N.; Levental, I. Remodeling of the postsynaptic plasma membrane during neural development. Mol. Biol. Cell 2016, 27, 3480–3489. [Google Scholar] [CrossRef] [Green Version]
- Levental, K.R.; Surma, M.A.; Skinkle, A.D.; Lorent, J.H.; Zhou, Y.; Klose, C.; Chang, J.T.; Hancock, J.F.; Levental, I. $ømega$-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Sci. Adv. 2017, 3, eaao1193. [Google Scholar] [CrossRef]
- Klose, C.; Surma, M.A.; Gerl, M.J.; Meyenhofer, F.; Shevchenko, A.; Simons, K. Flexibility of a Eukaryotic Lipidome – Insights from Yeast Lipidomics. PLoS ONE 2012, 7, e35063. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.A.; Klose, C.; Peng, D.; Shales, M.; Mrejen, C.; Stefanko, A.; Braberg, H.; Gordon, D.E.; Vorkel, D.; Ejsing, C.S.; et al. A Lipid E-MAP Identifies Ubx2 as a Critical Regulator of Lipid Saturation and Lipid Bilayer Stress. Mol. Cell 2013, 51, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Bakovic, M. Lipid rafts in health and disease. Biol. Cell 2007, 99, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, R.; Rojo, J.A.; Fabelo, N.; Fernandez, C.E.; Diaz, M. Lipid raft disarrangement as a result of neuropathological progresses: A novel strategy for early diagnosis? Neuroscience 2013, 245, 26–39. [Google Scholar] [CrossRef]
- Woodman, S.; Kim, K. Membrane Lipids: Implication for Diseases and Membrane Trafficking. SM J. Biol. 2017, 3, 1016. [Google Scholar]
- Evangelisti, E.; Cecchi, C.; Cascella, R.; Sgromo, C.; Becatti, M.; Dobson, C.M.; Chiti, F.; Stefani, M. Membrane lipid composition and its physicochemical properties define cell vulnerability to aberrant protein oligomers. J. Cell Sci. 2012, 125, 2416–2427. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; St. Clair, J.R.; London, E.; Raleigh, D.P. Islet Amyloid Polypeptide Membrane Interactions: Effects of Membrane Composition. Biochemistry 2017, 56, 376–390. [Google Scholar] [CrossRef] [Green Version]
- Soria, M.A.; Cervantes, S.A.; Bajakian, T.H.; Siemer, A.B. The Functional Amyloid Orb2A Binds to Lipid Membranes. Biophys. J. 2017, 113, 37–47. [Google Scholar] [CrossRef]
- Brown, A.M.; Bevan, D.R. Influence of sequence and lipid type on membrane perturbation by human and rat amyloid β-peptide (1–42). Arch. Biochem. Biophys. 2017, 614, 1–13. [Google Scholar] [CrossRef]
- Gunasekara, L.; Al-Saiedy, M.; Green, F.; Pratt, R.; Bjornson, C.; Yang, A.; Schoel, W.M.; Mitchell, I.; Brindle, M.; Montgomery, M.; et al. Pulmonary surfactant dysfunction in pediatric cystic fibrosis: Mechanisms and reversal with a lipid-sequestering drug. J. Cyst. Fibros. 2017, 16, 565–572. [Google Scholar] [CrossRef]
- Grassmé, H.; Henry, B.; Ziobro, R.; Becker, K.A.; Riethmüller, J.; Gardner, A.; Seitz, A.P.; Steinmann, J.; Lang, S.; Ward, C.; et al. β1-Integrin Accumulates in Cystic Fibrosis Luminal Airway Epithelial Membranes and Decreases Sphingosine, Promoting Bacterial Infections. Cell Host Microbe 2017, 21, 707–718.e8. [Google Scholar] [CrossRef] [PubMed]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantley, L.C.; Neel, B.G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 4240–4245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Ying, H.; Yan, H.; Kimmelman, A.C.; Hiller, D.J.; Chen, A.-J.; Perry, S.R.; Tonon, G.; Chu, G.C.; Ding, Z.; et al. P53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature 2008, 455, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Martin, M.L.; de Almeida, R.F.M.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; Guardiola-Serrano, F.; Lüth, A.; Kleuser, B.; Halver, J.E.; Escribá, P.V. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 19569–19574. [Google Scholar] [Green Version]
- Teres, S.; Llado, V.; Higuera, M.; Barcelo-Coblijn, G.; Martin, M.L.; Noguera-Salva, M.A.; Marcilla-Etxenike, A.; Garcia-Verdugo, J.M.; Soriano-Navarro, M.; Saus, C.; et al. 2-Hydroxyoleate, a nontoxic membrane binding anticancer drug, induces glioma cell differentiation and autophagy. Proc. Natl. Acad. Sci. USA 2012, 109, 8489–8494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuya, H.; Shimizu, Y.; Kawamori, T. Sphingolipids in cancer. Cancer Metastasis Rev. 2011, 30, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Obeid, L.M.; Linardic, C.M.; Karolak, L.A.; Hannun, Y.A. Programmed cell death induced by ceramide. Science 1993, 259, 1769–1771. [Google Scholar] [CrossRef]
- Venable, M.E.; Lee, J.Y.; Smyth, M.J.; Bielawska, A.; Obeid, L.M. Role of Ceramide in Cellular Senescence. J. Biol. Chem. 1995, 270, 30701–30708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hla, T. Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 2004, 15, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, J.C.; Turner, L.S.; Elojeimy, S.; Beckham, T.H.; Bielawska, A.; Keane, T.E.; Hannun, Y.A.; Norris, J.S. Acid ceramidase upregulation in prostate cancer: role in tumor development and implications for therapy. Expert Opin. Ther. Targets 2009, 13, 1449–1458. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Joshi, A.; Zhang, Y.; Jaeger, S.A.; Bulyk, M.; Tsichlis, P.N.; Shirley Liu, X.; Struhl, K. A Transcriptional Signature and Common Gene Networks Link Cancer with Lipid Metabolism and Diverse Human Diseases. Cancer Cell 2010, 17, 348–361. [Google Scholar] [CrossRef]
- Kuhajda, F.P.; Jenner, K.; Wood, F.D.; Hennigar, R.A.; Jacobs, L.B.; Dick, J.D.; Pasternack, G.R. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc. Natl. Acad. Sci. USA 1994, 91, 6379–6383. [Google Scholar] [CrossRef]
- Hilvo, M.; Denkert, C.; Lehtinen, L.; Muller, B.; Brockmoller, S.; Seppanen-Laakso, T.; Budczies, J.; Bucher, E.; Yetukuri, L.; Castillo, S.; et al. Novel Theranostic Opportunities Offered by Characterization of Altered Membrane Lipid Metabolism in Breast Cancer Progression. Cancer Res. 2011, 71, 3236–3245. [Google Scholar] [CrossRef] [Green Version]
- Crous-Bou, M.; Rennert, G.; Salazar, R.; Rodriguez-Moranta, F.; Rennert, H.S.; Lejbkowicz, F.; Kopelovich, L.; Lipkin, S.M.; Gruber, S.B.; Moreno, V. Genetic polymorphisms in fatty acid metabolism genes and colorectal cancer. Mutagenesis 2012, 27, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Dielschneider, R.F.; Henson, E.S.; Gibson, S.B. Lysosomes as Oxidative Targets for Cancer Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Park, J.B.; Lee, C.S.; Jang, J.-H.; Ghim, J.; Kim, Y.-J.; You, S.; Hwang, D.; Suh, P.-G.; Ryu, S.H. Phospholipase signalling networks in cancer. Nat. Rev. Cancer 2012, 12, 782–792. [Google Scholar] [CrossRef]
- Nomura, D.K.; Long, J.Z.; Niessen, S.; Hoover, H.S.; Ng, S.-W.; Cravatt, B.F. Monoacylglycerol Lipase Regulates a Fatty Acid Network that Promotes Cancer Pathogenesis. Cell 2010, 140, 49–61. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Santolla, M.F.; Lappano, R.; Marco, P.D.; Pupo, M.; Vivacqua, A.; Sisci, D.; Abonante, S.; Iacopetta, D.; Cappello, A.R.; Dolce, V.; et al. G Protein-coupled Estrogen Receptor Mediates the Up-regulation of Fatty Acid Synthase Induced by 17β-Estradiol in Cancer Cells and Cancer-associated Fibroblasts. J. Biol. Chem. 2012, 287, 43234–43245. [Google Scholar] [CrossRef]
- Im, S.-S.; Yousef, L.; Blaschitz, C.; Liu, J.Z.; Edwards, R.A.; Young, S.G.; Raffatellu, M.; Osborne, T.F. Linking Lipid Metabolism to the Innate Immune Response in Macrophages through Sterol Regulatory Element Binding Protein-1a. Cell Metab. 2011, 13, 540–549. [Google Scholar] [CrossRef]
- Heusinkveld, M.; de Vos van Steenwijk, P.J.; Goedemans, R.; Ramwadhdoebe, T.H.; Gorter, A.; Welters, M.J.P.; van Hall, T.; van der Burg, S.H. M2 Macrophages Induced by Prostaglandin E2 and IL-6 from Cervical Carcinoma Are Switched to Activated M1 Macrophages by CD4+ Th1 Cells. J. Immunol. 2011, 187, 1157–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Reference, G.H. Tay-Sachs Disease. Available online: https://ghr.nlm.nih.gov/condition/tay-sachs-disease (accessed on 25 April 2019).
- Marcilla-Etxenike, A.; Martín, M.L.; Noguera-Salvà, M.A.; García-Verdugo, J.M.; Soriano-Navarro, M.; Dey, I.; Escribá, P.V.; Busquets, X. 2-Hydroxyoleic Acid Induces ER Stress and Autophagy in Various Human Glioma Cell Lines. PLoS ONE 2012, 7, e48235. [Google Scholar] [CrossRef]
- Perona, J.S.; Vogler, O.; Sanchez-Dominguez, J.M.; Montero, E.; Escriba, P.V.; Ruiz-Gutierrez, V. Consumption of Virgin Olive Oil Influences Membrane Lipid Composition and Regulates Intracellular Signaling in Elderly Adults With Type 2 Diabetes Mellitus. J. Gerontol. A. Biol. Sci. Med. Sci. 2007, 62, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Escribá, P.V.; González-Ros, J.M.; Goñi, F.M.; Kinnunen, P.K.J.; Vigh, L.; Sánchez-Magraner, L.; Fernández, A.M.; Busquets, X.; Horváth, I.; Barceló-Coblijn, G. Membranes: a meeting point for lipids, proteins and therapies. J. Cell. Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wali, R.K.; Jaffe, S.; Kumar, D.; Kalra, V.K. Alterations in Organization of Phospholipids in Erythrocytes as Factor in Adherence to Endothelial Cells in Diabetes Mellitus. Diabetes 1988, 37, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018, 27, 22–41. [Google Scholar] [CrossRef]
- Basseri, S.; Austin, R.C. Endoplasmic Reticulum Stress and Lipid Metabolism: Mechanisms and Therapeutic Potential. Biochem. Res. Int. 2012, 2012. [Google Scholar] [CrossRef]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Lin, X.; Watkins, S.M.; Ivanov, A.R.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Settembre, C.; De Cegli, R.; Mansueto, G.; Saha, P.K.; Vetrini, F.; Visvikis, O.; Huynh, T.; Carissimo, A.; Palmer, D.; Jürgen Klisch, T.; et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 2013, 15, 647–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöneberg, J.; Lehmann, M.; Ullrich, A.; Posor, Y.; Lo, W.-T.; Lichtner, G.; Schmoranzer, J.; Haucke, V.; Noé, F. Lipid-mediated PX-BAR domain recruitment couples local membrane constriction to endocytic vesicle fission. Nat. Commun. 2017, 8, 15873. [Google Scholar] [CrossRef]
- Gray, S.M.; Aylor, K.W.; Barrett, E.J. Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia 2017, 60, 1512–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapani, L. Regulation and deregulation of cholesterol homeostasis: The liver as a metabolic “power station”. World J. Hepatol. 2012, 4, 184. [Google Scholar] [CrossRef]
- Radic, M.; Pattanaik, D. Cellular and Molecular Mechanisms of Anti-Phospholipid Syndrome. Front. Immunol. 2018, 9, 969. [Google Scholar] [CrossRef]
- Asherson, R.A.; Cervera, R. Antiphospholipid antibodies and infections. Ann. Rheum. Dis. 2003, 62, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Shahine, A.; Van Rhijn, I.; Cheng, T.-Y.; Iwany, S.; Gras, S.; Moody, D.B.; Rossjohn, J. A molecular basis of human T cell receptor autoreactivity toward self-phospholipids. Sci. Immunol. 2017, 2, eaao1384. [Google Scholar] [CrossRef]
- Palsuledesai, C.C.; Distefano, M.D. Protein prenylation: Enzymes, therapeutics, and biotechnology applications. ACS Chem. Biol. 2015, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ghomashchi, F.; Zhang, X.; Liu, L.; Gelb, M.H. Binding of prenylated and polybasic peptides to membranes: affinities and intervesicle exchange. Biochemistry 1995, 34, 11910–11918. [Google Scholar] [CrossRef]
- Resh, M.D. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2006, 2, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Casey, P.J. Protein Prenylation: Molecular Mechanisms and Functional Consequences. Annu. Rev. Biochem. 1996, 65, 241–269. [Google Scholar] [CrossRef]
- Kamiya, Y.; Sakurai, A.; Tamura, S.; Takahashi, N.; Abe, K.; Tsuchiya, E.; Fukui, S.; Kitada, C.; Fujino, M. Structure of rhodotorucine A, a novel lipopeptide, inducing mating tube formation in Rhodosporidiumtoruloides. Biochem. Biophys. Res. Commun. 1978, 83, 1077–1083. [Google Scholar] [CrossRef]
- Farnsworth, C.C.; Wolda, S.L.; Gelb, M.H.; Glomset, J.A. Human lamin B contains a farnesylated cysteine residue. J. Biol. Chem. 1989, 264, 20422–20429. [Google Scholar] [PubMed]
- Wolda, S.L.; Glomset, J.A. Evidence for modification of lamin B by a product of mevalonic acid. J. Biol. Chem. 1988, 263, 5997–6000. [Google Scholar] [PubMed]
- Berndt, N.; Hamilton, A.D.; Sebti, S.M. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 2011, 11, 775–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pompliano, D.L.; Schaber, M.D.; Mosser, S.D.; Omer, C.A.; Shafer, J.A.; Gibbs, J.B. Isoprenoid diphosphate utilization by recombinant human farnesyl:protein transferase: Interactive binding between substrates and a preferred kinetic pathway. Biochemistry 1993, 32, 8341–8347. [Google Scholar] [CrossRef] [PubMed]
- Dolence, J.M.; Cassidy, P.B.; Mathis, J.R.; Poulter, C.D. Yeast protein farnesyltransferase: steady-state kinetic studies of substrate binding. Biochemistry 1995, 34, 16687–16694. [Google Scholar] [CrossRef]
- Omer, C.A.; Gibbs, R.A. On the Stereochemical Course of Human Protein-Farnesyl Transferase. J. Am. Chem. Soc. 1996, 118, 1817–1823. [Google Scholar]
- Edelstein, R.L.; Weller, V.A.; Distefano, M.D.; Tung, J.S. Stereochemical Analysis of the Reaction Catalyzed by Yeast Protein Farnesyltransferase. J. Org. Chem. 1998, 63, 5298–5299. [Google Scholar] [CrossRef]
- Clausen, V.A.; Edelstein, R.L.; Distefano, M.D. Stereochemical Analysis of the Reaction Catalyzed by Human Protein Geranylgeranyl Transferase †. Biochemistry 2001, 40, 3920–3930. [Google Scholar] [CrossRef] [PubMed]
- Long, S.B.; Casey, P.J.; Beese, L.S. Reaction path of protein farnesyltransferase at atomic resolution. Nature 2002, 419, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Ochocki, J.D.; Distefano, M.D. Prenyltransferase inhibitors: treating human ailments from cancer to parasitic infections. Med. Chem. Commun. 2013, 4, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Desnoyers, L.; Anant, J.S.; Seabra, M.C. Geranylgeranylation of Rab proteins. Biochem. Soc. Trans. 2015, 24, 699–703. [Google Scholar] [CrossRef]
- Bos, J.L. Ras oncogenes in human cancer: a review. Cancer Res. 1989, 49, 4682–4689. [Google Scholar]
- Bishop, W.R.; Doll, R.; Kirschmeier, P. Farnesyl Transferase Inhibitors. In Enzymes; Academic Press: Cambridge, MA, USA, 2011; pp. 275–303. [Google Scholar]
- Hamad, N.M. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002, 16, 2045–2057. [Google Scholar] [CrossRef] [Green Version]
- Vogt, A.; Sun, J.; Qian, Y.; Hamilton, A.D.; Sebti, S.M. The Geranylgeranyltransferase-I Inhibitor GGTI-298 Arrests Human Tumor Cells in G 0 /G 1 and Induces p21 WAF1/CIP1/SDI1 in a p53-independent Manner. J. Biol. Chem. 1997, 272, 27224–27229. [Google Scholar] [CrossRef]
- Mollinedo, F.; de la Iglesia-Vicente, J.; Gajate, C.; Estella-Hermoso de Mendoza, A.; Villa-Pulgarin, J.A.; Campanero, M.A.; Blanco-Prieto, M.J. Lipid raft-targeted therapy in multiple myeloma. Oncogene 2010, 29, 3748–3757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäumer, W.; Wlaź, P.; Jennings, G.; Rundfeldt, C. The putative lipid raft modulator miltefosine displays immunomodulatory action in T-cell dependent dermal inflammation models. Eur. J. Pharmacol. 2010, 628, 226–232. [Google Scholar] [CrossRef]
- Dölle, S.; Hoser, D.; Rasche, C.; Loddenkemper, C.; Maurer, M.; Zuberbier, T.; Worm, M. Long-term reduction in local inflammation by a lipid raft molecule in atopic dermatitis. Allergy 2010, 65, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Lladó, V.; López, D.J.; Ibarguren, M.; Alonso, M.; Soriano, J.B.; Escribá, P.V.; Busquets, X. Regulation of the cancer cell membrane lipid composition by NaCHOleate. Biochim. Biophys. Acta BBA - Biomembr. 2014, 1838, 1619–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.; Price, S.L.; Fiol-deRoque, M.A.; Marcilla-Etxenike, A.; Ahyayauch, H.; Barceló-Coblijn, G.; Terés, S.; Katsouri, L.; Ordinas, M.; López, D.J.; et al. Membrane lipid modifications and therapeutic effects mediated by hydroxydocosahexaenoic acid on Alzheimer’s disease. Biochim. Biophys. Acta BBA - Biomembr. 2014, 1838, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Ash, M.E. Lipid Replacement Therapy: A natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochim. Biophys. Acta BBA - Biomembr. 2014, 1838, 1657–1679. [Google Scholar] [CrossRef] [Green Version]
- Escriba, P.V.; Sastre, M.; Garcia-Sevilla, J.A. Disruption of cellular signaling pathways by daunomycin through destabilization of nonlamellar membrane structures. Proc. Natl. Acad. Sci. USA 1995, 92, 7595–7599. [Google Scholar] [CrossRef] [PubMed]
- Escriba, P.V.; Morales, P.; Smith, A. Membrane Phospholipid Reorganization Differentially Regulates Metallothionein and Heme Oxygenase by Heme–Hemopexin. DNA Cell Biol. 2002, 21, 355–364. [Google Scholar] [CrossRef]
- Vigh, L.; Maresca, B.; Harwood, J.L. Does the membrane’s physical state control the expression of heat shock and other genes? Trends Biochem. Sci. 1998, 23, 369–374. [Google Scholar] [CrossRef]
- Moulin, M.; Carpentier, S.; Levade, T.; Arrigo, A.-P. Potential roles of membrane fluidity and ceramide in hyperthermia and alcohol stimulation of TRAIL apoptosis. Apoptosis 2007, 12, 1703–1720. [Google Scholar] [CrossRef] [PubMed]
- Irvine, R.F. Nuclear lipid signalling. Nat. Rev. Mol. Cell Biol. 2003, 4, 349–361. [Google Scholar] [CrossRef]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta BBA - Biomembr. 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission Possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef] [Green Version]
- Moreira, R.A.; Mendanha, S.A.; Hansen, D.; Alonso, A. Interaction of Miltefosine with the Lipid and Protein Components of the Erythrocyte Membrane. J. Pharm. Sci. 2013, 102, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, P.; Rosselló, C.A.; Rodríguez-Lorca, R.; Beteta-Göbel, R.; Fernández-Díaz, J.; Lladó, V.; Busquets, X.; Escribá, P.V.; Fernández-García, P.; Rosselló, C.A.; et al. The Opposing Contribution of SMS1 and SMS2 to Glioma Progression and Their Value in the Therapeutic Response to 2OHOA. Cancers 2019, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Vögler, O.; Casas, J.; Barceló, F.; Alemany, R.; Prades, J.; Nagy, T.; Baamonde, C.; Kasprzyk, P.G.; Terés, S.; et al. Membrane Structure Modulation, Protein Kinase C Activation, and Anticancer Activity of Minerval. Mol. Pharmacol. 2005, 67, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Erazo, T.; Lorente, M.; López-Plana, A.; Muñoz-Guardiola, P.; Fernández-Nogueira, P.; García-Martínez, J.A.; Bragado, P.; Fuster, G.; Salazar, M.; Espadaler, J.; et al. The New Antitumor Drug ABTL0812 Inhibits the Akt/mTORC1 Axis by Upregulating Tribbles-3 Pseudokinase. Clin. Cancer Res. 2016, 22, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Serrano, F.; Beteta-Göbel, R.; Rodríguez-Lorca, R.; Ibarguren, M.; López, D.J.; Terés, S.; Alvarez, R.; Alonso-Sande, M.; Busquets, X.; Escribá, P.V. The Novel Anticancer Drug Hydroxytriolein Inhibits Lung Cancer Cell Proliferation via a Protein Kinase Cα– and Extracellular Signal-Regulated Kinase 1/2–Dependent Mechanism. J. Pharmacol. Exp. Ther. 2015, 354, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vellon, L.; Lupu, R. Antitumoral actions of the anti-obesity drug orlistat (XenicalTM) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene. Ann. Oncol. 2005, 16, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Vögler, O.; López-Bellan, A.; Alemany, R.; Tofé, S.; González, M.; Quevedo, J.; Pereg, V.; Barceló, F.; Escriba, P.V. Structure–effect relation of C18 long-chain fatty acids in the reduction of body weight in rats. Int. J. Obes. 2008, 32, 464–473. [Google Scholar] [CrossRef]
- Teres, S.; Barcelo-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escriba, P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.C.; Conklin, S.M.; Manuck, S.B.; Yao, J.K.; Muldoon, M.F. Long-Chain Omega-3 Fatty Acids and Blood Pressure. Am. J. Hypertens. 2011, 24, 1121–1126. [Google Scholar] [CrossRef]
- Alemany, R.; Terés, S.; Baamonde, C.; Benet, M.; Vögler, O.; Escribá, P.V. 2-Hydroxyoleic Acid. Hypertension 2004, 43, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Alemany, R.; Vögler, O.; Terés, S.; Egea, C.; Baamonde, C.; Barceló, F.; Delgado, C.; Jakobs, K.H.; Escribá, P.V. Antihypertensive action of 2-hydroxyoleic acid in SHRs via modulation of the protein kinase A pathway and Rho kinase. J. Lipid Res. 2006, 47, 1762–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchert, G.H.; Giggey, M.; Kolar, F.; Wong, T.M.; Backx, P.H.; Escriba, P.V. 2-Hydroxyoleic acid affects cardiomyocyte [Ca2+]i transient and contractility in a region-dependent manner. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H1948–H1955. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S. Neuroprotective and Ameliorative Actions of Polyunsaturated Fatty Acids Against Neuronal Diseases: Beneficial Effect of Docosahexaenoic Acid on Cognitive Decline in Alzheimer’s Disease. J. Pharmacol. Sci. 2011, 116, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.L.; Barceló-Coblijn, G.; de Almeida, R.F.M.; Noguera-Salvà, M.A.; Terés, S.; Higuera, M.; Liebisch, G.; Schmitz, G.; Busquets, X.; Escribá, P.V. The role of membrane fatty acid remodeling in the antitumor mechanism of action of 2-hydroxyoleic acid. Biochim. Biophys. Acta BBA - Biomembr. 2013, 1828, 1405–1413. [Google Scholar] [CrossRef] [Green Version]
- Fiol-deRoque, M.A.; Gutierrez-Lanza, R.; Terés, S.; Torres, M.; Barceló, P.; Rial, R.V.; Verkhratsky, A.; Escribá, P.V.; Busquets, X.; Rodríguez, J.J. Cognitive recovery and restoration of cell proliferation in the dentate gyrus in the 5XFAD transgenic mice model of Alzheimer’s disease following 2-hydroxy-DHA treatment. Biogerontology 2013, 14, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Avila-Martin, G.; Galan-Arriero, I.; Gómez-Soriano, J.; Taylor, J. Treatment of Rat Spinal Cord Injury with the Neurotrophic Factor Albumin-Oleic Acid: Translational Application for Paralysis, Spasticity and Pain. PLoS ONE 2011, 6, e26107. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092167
Casares D, Escribá PV, Rosselló CA. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. International Journal of Molecular Sciences. 2019; 20(9):2167. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092167
Chicago/Turabian StyleCasares, Doralicia, Pablo V. Escribá, and Catalina Ana Rosselló. 2019. "Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues" International Journal of Molecular Sciences 20, no. 9: 2167. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092167