Establishment and Characterization of a Murine Mucosal Mast Cell Culture Model
Abstract
:1. Introduction
2. Results
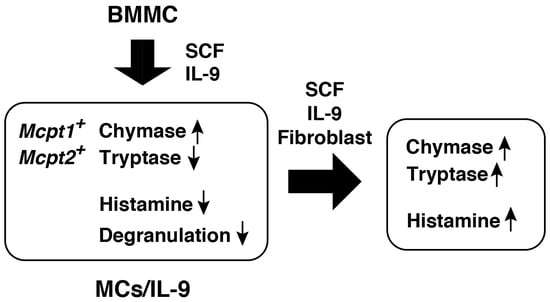
2.1. Combination of IL-9 and SCF Induced Expression of Mcpt1 and Mcpt2 and Depleted Histamine in Murine BMMCs
2.2. Antigen-Induced Degranulation Was Attenuated in MCs/IL-9 When They Were Sensitized with Lower Concentrations of IgE
2.3. Degranulation of MCs/IL-9 in Response to ATP
2.4. Induction of the Inflammatory Cytokine Gene Expression in MCs/IL-9
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of Bone-Marrow-Derived Cultured Mast Cells
4.4. Flowcytometry
4.5. Measurement of Granule Protease Activities
4.6. Measurement of Enzymatic Activity of Histidine Decarboxylase
4.7. Measurement of Degranulation
4.8. Quantitative RT-PCR
4.9. Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BMMC | IL-3-dependent bone-marrow-derived cultured mast cell |
| FBS | Fetal bovine serum |
| MC/IL-9 | IL-9-modified mast cell |
| PCR | Polymerase chain reaction |
| SCF | Stem cell factor (c-kit ligand) |
References
- Kitamura, Y. Heterogeneity of mast cells and phenotypic changes between subpopulations. Annu. Rev. Immunol. 1989, 7, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Grimbaldeston, M.; Tsai, M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodbury, R.G.; Miller, H.R.; Huntley, J.F.; Newlands, G.F.; Palliser, A.C.; Wakelin, D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. Nature 1984, 312, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat. Rev. Immunol. 2007, 7, 93–104. [Google Scholar] [CrossRef]
- Albert-Bayo, M.; Paracuellos, I.; González-Castro, A.M.; Rodriguez-Urrutia, A.; Rodríguez-Lagunas, M.J.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Intestinal mucosal mast cells: key modulators of barrier function and homeostasis. Cells 2019, 8, E135. [Google Scholar] [CrossRef] [Green Version]
- Hültner, L.; Druez, C.; Moeller, J.; Uyttenhove, C.; Schmitt, E.; Rüde, E.; Dörmer, P.; van Snick, J. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9). Eur. J. Immunol. 1990, 20, 1413–1416. [Google Scholar] [CrossRef]
- Faulkner, H.; Humphreys, N.; Renauld, J.C.; Van Snick, J.; Grencis, R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur. J. Immunol. 1997, 27, 2536–2540. [Google Scholar] [CrossRef]
- Godfrained, C.; Louahed, J.; Faulkner, H.; Vink, A.; Warnier, G.; Grencis, R.; Renauld, J.C. Intraepithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. J. Immunol. 1998, 160, 3989–3996. [Google Scholar]
- Faulkner, H.; Renauld, J.C.; Van Snick, J.; Grencis, R.K. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect. Immun. 1998, 66, 3832–3840. [Google Scholar]
- Townsend, J.M.; Fallon, G.P.; Matthews, J.D.; Smith, P.; Jolin, E.H.; McKenzie, N.A. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 2000, 13, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Osterfeld, H.; Ahrens, R.; Strait, R.; Finkelman, F.D.; Renauld, J.C.; Hogan, S.P. Differential roles for the IL-9/IL-9 receptor a-chain pathway in systemic and oral antigen–induced anaphylaxis. J. Allergy Clin. Immunol. 2010, 125, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eklund, K.K.; Ghildyal, F.; Austen, K.F.; Stevens, R.L. Induction by IL-9 and suppression by IL-3 and IL-4 of the levels of chromosome 14-derived transcripts that encode late-expressed mouse mast cell proteases. J. Immunol. 1993, 151, 4266–4273. [Google Scholar] [PubMed]
- Nakahata, T.; Kobayashi, T.; Ishuguro, A.; Tsuji, K.; Naganuma, K.; Ando, O.; Yagi, Y.; Tadokoro, K.; Akabane, T. Extensive proliferation of mature connective-tissue type mast cells in vitro. Nature 1986, 324, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Lantz, C.S.; Boesiger, J.; Song, C.H.; Mach, N.; Kobayashi, T.; Mulligan, R.C.; Nawa, Y.; Dranoff, G.; Galli, S.J. Role for interleukin-3 in mast cell and basophil development and in immunity to parasites. Nature 1998, 392, 90–93. [Google Scholar] [CrossRef]
- Scudamore, C.L.; McMillan, L.; Thornton, E.M.; Wright, S.H.; Newlands, G.F.J.; Miller, H.R.P. Mast cell heterogeneity in the gastrointestinal tract: variable expression of mouse mast cell protease-1 (mMCP-1) in intraepithelial mucosal mast cells in nematode-infected and normal BALB/c mice. Am. J. Pathol. 1997, 150, 1661–1672. [Google Scholar]
- Reber, L.L.; Marichal, T.; Galli, S.J. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012, 33, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Takano, H.; Nakazawa, S.; Okuno, Y.; Shirata, N.; Tsuchiya, S.; Kainoh, T.; Takamatsu, S.; Furuta, K.; Taketomi, Y.; Naito, Y.; et al. Establishment of the culture model system that reflects the process of terminal differentiation of connective tissue-type mast cells. FEBS Lett. 2008, 582, 1444–1450. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Sato, H.; Sakamaki, K.; Kamada, M.; Okuno, Y.; Fukuishi, N.; Furuta, K.; Tanaka, S. Suppression of IgE-independent degranulation of murine connective tissue-type mast cells by dexamethasone. Cells 2019, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, S.; Tachida, Y.; Segi-Nishida, E.; Okuno, Y.; Tamba, S.; Tsujimoto, G.; Tanaka, S.; Sugimoto, Y. Characterization of gene expression profiles for different types of mast cells pooled from mouse stomach subregions by an RNA amplification method. BMC Genom. 2009, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.G.; Jacobson, K.A. Purinergic signaling in mast cell degranulation and asthma. Front. Pharmacol. 2017, 8, 947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Ito, M.; Hoshino, Y.; Matsuoka, I. Effects of dexamethasone on purinergic signaling in murine mast cells: Selective suppression of P2X7 receptor expression. Biochem. Biophys. Res. Commun. 2017, 493, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.R.; Galli, S.J. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J. Exp. Med. 1991, 174, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andoh, A.; Deguchi, Y.; Inatomi, O.; Yagi, Y.; Bamba, S.; Tsujikawa, T.; Fujiyama, Y. Immunohistochemical study of chymase-positive mast cells in inflammatory bowel disease. Oncol. Rep. 2006, 16, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Lu, X.; Jia, X.; Zhou, T.; Guo, C. Soluble mediators released from PI-IBS patients’ colon induced alteration of mast cells: involvement of reactive oxygen species. Dig. Dis. Sci. 2012, 57, 311–319. [Google Scholar] [CrossRef]
- De Winter, B.Y.; van den Wijngaard, R.M.; de Jonge, W.J. Intestinal mast cells in gut inflammation and motility disturbances. Biochim. Biophys. Acta 2012, 1822, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Wouters, M.M.; Vicario, M.; Santos, J. The role of mast cells in functional GI disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, S.C. Mast cells in gastrointestinal disorders. Eur. J. Pharmacol. 2016, 778, 139–145. [Google Scholar] [CrossRef]
- Tsai, M.; Takeishi, T.; Thompson, H.; Langley, K.E.; Zsebo, K.M.; Metcalfe, D.D.; Geissler, E.N.; Galli, S.J. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc. Natl. Acad. Sci. USA 1991, 88, 6382–6386. [Google Scholar] [CrossRef] [Green Version]
- Mortillaro, N.A.; Granger, D.N.; Kvietys, P.R.; Rutill, G.; Taylor, A.E. Effects of histamine and histamine antagonists on intestinal capillary permeability. Am. J. Physiol. 1981, 240, G381–G386. [Google Scholar] [CrossRef]
- Keely, S.J.; Stack, W.A.; O’Donoghue, D.P.; Baird, A.W. Regulation of ion transport by histamine in human colon. Eur. J. Pharmacol. 1995, 279, 203–209. [Google Scholar] [CrossRef]
- Wechsler, J.B.; Szabo, A.; Hsu, C.L.; Krier-Burris, R.A.; Schroeder, H.A.; Wang, M.Y.; Carter, R.G.; Velez, T.E.; Aguiniga, L.M.; Brown, J.B.; et al. Histamine drives severity of innate inflammation via histamine 4 receptor in murine experimental colitis. Mucosal Immunol. 2018, 11, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forward, N.A.; Furlong, S.J.; Yang, Y.; Lin, T.J.; Hoskin, D.W. Mast cells down-regulate CD4+CD25+ T reguratory cell suppressor function via histamine H1 receptor interaction. J. Immunol. 2009, 183, 3014–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, E.E.; Groschwitz, K.; Abonia, J.P.; Brandt, E.B.; Cohen, E.; Blanchard, C.; Ahrens, R.; Seidu, L.; McKenzie, A.; Strait, R.; et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J. Exp. Med. 2008, 205, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.S.; Ghildyal, N.; Gurish, M.F.; Hunt, J.; Hu, X.; Austen, K.F.; Stevens, R.L. Reversible expression of tryptases and chymases in the jejunum mast cells of mice infected with Trichinella spiralis. J. Immunol. 1998, 160, 5537–5545. [Google Scholar]
- Knight, P.A.; Wright, S.H.; Lawrence, C.E.; Paterson, Y.Y.W.; Miller, H.R.P. Delayed expulsion of the Nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 2000, 192, 1849–1856. [Google Scholar] [CrossRef] [Green Version]
- Renga, G.; Moretti, S.; Oikonomou, V.; Borghi, M.; Zelente, T.; Paolicelli, G.; Costantini, C.; De Zuani, M.; Villella, V.R.; Raia, V.; et al. IL-9 and mast cells are key players of Candida albicans commensalism and pathogenesis in the gut. Cell Rep. 2018, 23, 1767–1778. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, M.J.; Sinnamon, M.J.; Lyng, G.D.; Glickman, J.N.; Wang, X.; Xing, W.; Krilis, S.A.; Blumberg, R.S.; Adachi, R.; Lee, D.M.; et al. Essential role for mast cell tryptase in acute experimental colitis. Proc. Natl. Acad. Sci. USA 2011, 108, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Sehra, S.; Yao, W.; Nguyen, E.T.; Glosson-Byers, N.L.; Akhtar, N.; Zhou, B.; Kaplan, M.H. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J. Allergy Clin. Immunol. 2015, 136, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.F.; Lind, E.F.; Gondek, D.C.; Bennett, K.A.; Gleeson, M.W.; Pino-Lagos, K.; Scott, Z.A.; Coyle, A.J.; Reed, J.L.; Van Snick, J.; et al. Mast cells are essentials intermediaries in regulatory T-cell tolerance. Nature 2006, 442, 997–1002. [Google Scholar] [CrossRef]
- Hültner, L.; Kölsch, S.; Stassen, M.; Kaspers, U.; Kremer, J.P.; Mailhammer, R.; Moeller, J.; Broszeit, H.; Schmitt, E. In activated mast cells, IL-1 up-regulates the production of several Th2-related cytokines including IL-9. J. Immunol. 2000, 164, 5556–5563. [Google Scholar] [CrossRef] [PubMed]
- Stassen, M.; Arnold, M.; Hültner, L.; Müller, C.; Neudörfl, C.; Reineke, T.; Schmitt, E. Murine bone marrow-derived mast cells as potent producers of IL-9: Costimulatory function of IL-10 and kit ligand in the presence of IL-1. J. Immunol. 2000, 164, 5549–5555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stassen, M.; Müller, C.; Arnold, M.; Hültner, L.; Klein-Hessling, S.; Neudörfl, C.; Reineke, T.; Serfling, E.; Schmitt, E. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-kappa B is decisively involved in the expression of IL-9. J. Immunol. 2001, 166, 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lee, J.B.; Liu, B.; Ohta, S.; Wang, P.Y.; Kartashov, A.V.; Mugge, L.; Abonia, J.P.; Barski, A.; Izuhara, K.; et al. Induction of interleukin-9-producing mucosal mast cells promotes susceptibility to IgE-mediated experimental food allergy. Immunity 2015, 43, 788–802. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.G.; Hallgren, J.; Humbles, A.; Burwell, T.; Finkelman, F.D.; Alcaide, P.; Austen, K.F.; Gurish, M.F. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restircted NKT cells. J. Immunol. 2009, 183, 5251–5260. [Google Scholar] [CrossRef]
- Kurashima, Y.; Amiya, T.; Nohi, T.; Fujisawa, K.; Haraguchi, T.; Iba, H.; Tsutsui, H.; Sato, S.; Nakajima, S.; Iijima, H.; et al. Extracelluar ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoreceptors. Nat. Cummun. 2012, 3, 1034. [Google Scholar] [CrossRef]
- Tanaka, S.; Takasu, Y.; Mikura, S.; Satoh, N.; Ichikawa, A. Antigen-independent induction of histamine synthesis by immunoglobulin E in mouse bone marrow-derived mast cells. J. Exp. Med. 2002, 196, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Yamatodani, A.; Fukuda, H.; Wada, H.; Iwaeda, T.; Watanabe, T. High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: Cation-exchange chromatography coupled with post-column derivatization fluorometry. J. Chrmatogr. 1985, 344, 115–123. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakinoki, A.; Kameo, T.; Yamashita, S.; Furuta, K.; Tanaka, S. Establishment and Characterization of a Murine Mucosal Mast Cell Culture Model. Int. J. Mol. Sci. 2020, 21, 236. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010236
Kakinoki A, Kameo T, Yamashita S, Furuta K, Tanaka S. Establishment and Characterization of a Murine Mucosal Mast Cell Culture Model. International Journal of Molecular Sciences. 2020; 21(1):236. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010236
Chicago/Turabian StyleKakinoki, Aya, Tsuyoshi Kameo, Shoko Yamashita, Kazuyuki Furuta, and Satoshi Tanaka. 2020. "Establishment and Characterization of a Murine Mucosal Mast Cell Culture Model" International Journal of Molecular Sciences 21, no. 1: 236. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010236