Self-Assembly in Ganglioside‒Phospholipid Systems: The Co-Existence of Vesicles, Micelles, and Discs
Abstract
:1. Introduction
2. Results
2.1. Lipid Molecular Dynamics in DOPC-GM1 Mixtures
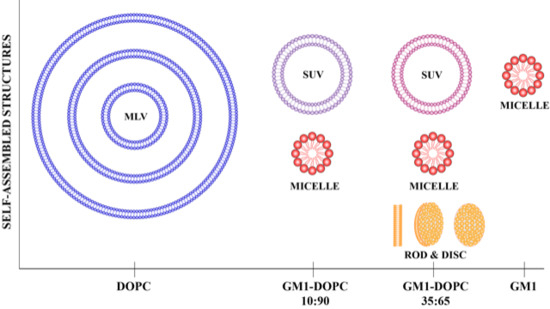
2.2. Cryo-TEM Observations of DOPC-GM1 Mixtures
2.3. Scattering and NMR Characterization of GM1 Micelles and DOPC-GM1 Mixtures
2.4. Temperature-Induced Changes in the GM1-Water System
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Cryogenic Transmission Electron Microscopy (cryo-TEM)
4.4. Small-Angle X-Ray Scattering (SAXS)
4.5. Differential Scanning Calorimetry (DSC)
4.6. Dynamic Light Scattering (DLS)
4.7. Polarization Transfer Solid State NMR (PTssNMR)
4.8. Diffusion NMR
4.9. 31P NMR
4.10. Nnano-ESI-MS
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GM | Ganglioside mono-sialylated |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| PTssNMR | Polarization transfer solid state nuclear magnetic resonance |
| MAS | Magic angle spinning |
| CP | Cross-polarization |
| DP | Direct polarization |
| INEPT | Insensitive nuclei enhanced by polarization transfer |
| AT | All trans |
| TG | Trans/gauche |
| PFG-STE | Pulsed field gradient stimulated echo |
| TEM | Transmission electron microscopy |
| SAXS | Small-angle X-ray scattering |
| ESI MS | Electro spray ionization mass spectrometry |
| DLS | Dynamic light scattering |
| DSC | Differential scanning calorimetry |
| CMC | Critical micellar concentration |
References
- Aureli, M.; Mauri, L.; Ciampa, M.G.; Prinetti, A.; Toffano, G.; Secchieri, C.; Sonnino, S. GM1 Ganglioside: Past Studies and Future Potential. Mol. Neurobiol. 2016, 53, 1824–1842. [Google Scholar] [CrossRef] [PubMed]
- Malisan, F.; Testi, R. GD3 ganglioside and apoptosis. Biochim. Biophys. Acta 2002, 1585, 179–187. [Google Scholar] [CrossRef]
- Sonnino, S.; Mauri, L.; Ciampa, M.G.; Prinetti, A. Gangliosides as regulators of cell signaling: Ganglioside-protein interactions or ganglioside-driven membrane organization? J. Neurochem. 2013, 124, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Kolter, T. Ganglioside biochemistry. ISRN Biochem. 2012, 2012, 506160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kracun, I.; Rosner, H.; Cosovic, C.; Stavljenic, A. Topographical atlas of the gangliosides of the adult human brain. J. Neurochem. 1984, 43, 979–989. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Yu, R.K. Gangliosides: Structure, isolation, and analysis. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1982; Volume 83, pp. 139–191. [Google Scholar]
- Yu, R.K.; Nakatani, Y.; Yanagisawa, M. The role of glycosphingolipid metabolism in the developing brain. J. Lipid Res. 2009, 50, S440–S445. [Google Scholar] [CrossRef] [Green Version]
- Grey, M.; Dunning, C.J.; Gaspar, R.; Grey, C.; Brundin, P.; Sparr, E.; Linse, S. Acceleration of alpha-synuclein aggregation by exosomes. J. Biol. Chem. 2015, 290, 2969–2982. [Google Scholar] [CrossRef] [Green Version]
- Ariga, T.; McDonald, M.P.; Yu, R.K. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease-a review. J. Lipid Res. 2008, 49, 1157–1175. [Google Scholar] [CrossRef] [Green Version]
- Cavdarli, S.; Groux-Degroote, S.; Delannoy, P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules 2019, 9, 311. [Google Scholar] [CrossRef] [Green Version]
- Forsayeth, J.; Hadaczek, P. Ganglioside Metabolism and Parkinson’s Disease. Front. Neurosci. 2018, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Klenk, E. Niemann-Pick’sche Krankheitund Amaurotische Idiotie. Hoppe-Seyler’s Z. Physiol. Chem. 1939, 262, 128–143. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Wu, G. Chapter Fifteen-Gangliosides, α-Synuclein, and Parkinson’s Disease. In Progress in Molecular Biology and Translational Science; Schnaar, R.L., Lopez, P.H.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 156, pp. 435–454. [Google Scholar]
- O’Brien, J.S. Ganglioside Storage Diseases. In Advances in Human Genetics; Harris, H., Hirschhorn, K., Eds.; Springer US: Boston, MA, USA, 1972; pp. 39–98. [Google Scholar]
- Sandhoff, K.; Harzer, K. Gangliosides and Gangliosidoses: Principles of Molecular and Metabolic Pathogenesis. J. Neurosci. 2013, 33, 10195. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K. GM1 ganglioside and Alzheimer’s disease. Glycoconj. J. 2015, 32, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Klenk, E. Über die Natur der Phosphatide und anderer Lipide des Gehirns und der Leber bei der Niemann-Pick’schen Krankheit. Z. Für. Physiol. Chem. 1935, 235, 24–25. [Google Scholar] [CrossRef]
- Klenk, E. Die Fettstoffe des Gehirnsbei Amaurotischer Idiotie und Niemann-Pick’scher Krankheit. Ber. Ges. Physiol. 1937, 96, 659–660. [Google Scholar]
- Klenk, E. Über die Ganglioside, eine neue Gruppe vonzuckerhaltigen Gehirnlipoiden. Hoppe-Seyler’s Z. Für Physiol. Chem. 1942, 273, 76–86. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [Green Version]
- Svennerholm, L. Chromatographic separation of human brain gangliosides. J. Neurochem. 1963, 10, 613–623. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Wu, G. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem. Sci. 2015, 40, 407–418. [Google Scholar] [CrossRef]
- Bolot, G.; David, M.J.; Taki, T.; Handa, S.; Kasama, T.; Richard, M.; Pignat, J.C.; Thomas, L.; Portoukalian, J. Analysis of glycosphingolipids of human head and neck carcinomas with comparison to normal tissue. Biochem. Mol. Biol. Int. 1998, 46, 125–135. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: San Diego, CA, USA, 2011. [Google Scholar]
- Cantu, L.; Del Favero, E.; Brocca, P.; Corti, M. Multilevel structuring of ganglioside-containing aggregates: From simple micelles to complex biomimetic membranes. Adv. Colloid Interface Sci. 2014, 205, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.L.; Chi, E.Y.; Arratia, C.; Majewski, J.; Kjaer, K.; Lee, K.Y. Condensing and fluidizing effects of ganglioside GM1 on phospholipid films. Biophys. J. 2008, 94, 3047–3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedlovszky, P.; Sega, M.; Vallauri, R. GM1 Ganglioside Embedded in a Hydrated DOPC Membrane: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2009, 113, 4876–4886. [Google Scholar] [CrossRef] [PubMed]
- Sillerud, L.O.; Schafer, D.E.; Yu, R.K.; Konigsberg, W.H. Calorimetric properties of mixtures of ganglioside GM1 and dipalmitoylphosphatidylcholine. J. Biol. Chem. 1979, 254, 10876–10880. [Google Scholar] [PubMed]
- Regina Todeschini, A.; Hakomori, S.-i. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta 2008, 1780, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Rodi, P.M.; Maggio, B.; Bagatolli, L.A. Direct visualization of the lateral structure of giant vesicles composed of pseudo-binary mixtures of sulfatide, asialo-GM1 and GM1 with POPC. Biochim. Biophys. Acta. Biomembr. 2018, 1860, 544–555. [Google Scholar] [CrossRef]
- Hirai, M.; Sato, S.; Kimura, R.; Hagiwara, Y.; Kawai-Hirai, R.; Ohta, N.; Igarashi, N.; Shimizu, N. Effect of protein-encapsulation on thermal structural stability of liposome composed of glycosphingolipid/cholesterol/phospholipid. J. Phys. Chemistry. B 2015, 119, 3398–3406. [Google Scholar] [CrossRef]
- Shi, J.; Yang, T.; Kataoka, S.; Zhang, Y.; Diaz, A.J.; Cremer, P.S. GM1 clustering inhibits cholera toxin binding in supported phospholipid membranes. J. Am. Chem. Soc. 2007, 129, 5954–5961. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, T.; Nishihara, M.; Yasumori, H.; Nakai, M.; Yanagisawa, K.; Sato, T. Size and Shape of Amyloid Fibrils Induced by Ganglioside Nanoclusters: Role of Sialyl Oligosaccharide in Fibril Formation. Langmuir 2017, 33, 13874–13881. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Kato, K.; Yanagisawa, K. Abeta polymerization through interaction with membrane gangliosides. Biochim. Biophys. Acta 2010, 1801, 868–877. [Google Scholar] [CrossRef]
- Frey, S.L.; Lee, K.Y.C. Number of sialic acid residues in ganglioside headgroup affects interactions with neighboring lipids. Biophys. J. 2013, 105, 1421–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, R.; Pallbo, J.; Weininger, U.; Linse, S.; Sparr, E. Ganglioside lipids accelerate α-synuclein amyloid formation. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, A.; Mohr, P.C.; Norrman, J.; Martin, R.W.; Topgaard, D. Polarization transfer solid-state NMR for studying surfactant phase behavior. Langmuir 2010, 26, 16848–16856. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, A.; Bongartz, N.A.; Ollila, O.H.S.; Nylander, T.; Topgaard, D. Signal intensities in 1H–13C CP and INEPT MAS NMR of liquid crystals. J. Magn. Reson. 2013, 230, 165–175. [Google Scholar] [CrossRef]
- Nowacka, A.; Douezan, S.; Wadsö, L.; Topgaard, D.; Sparr, E. Small polar molecules like glycerol and urea can preserve the fluidity of lipid bilayers under dry conditions. Soft Matter 2012, 8, 1482–1491. [Google Scholar] [CrossRef]
- Galvagnion, C.; Topgaard, D.; Makasewicz, K.; Buell, A.K.; Linse, S.; Sparr, E.; Dobson, C.M. Lipid Dynamics and Phase Transition within α-Synuclein Amyloid Fibrils. J. Phys. Chem. Lett. 2019, 10, 7872–7877. [Google Scholar] [CrossRef] [Green Version]
- Hellstrand, E.; Nowacka, A.; Topgaard, D.; Linse, S.; Sparr, E. Membrane lipid co-aggregation with α-synuclein fibrils. PLoS ONE 2013, 8, e77235. [Google Scholar] [CrossRef] [Green Version]
- Andersson, J.M.; Grey, C.; Larsson, M.; Ferreira, T.M.; Sparr, E. Effect of cholesterol on the molecular structure and transitions in a clinical-grade lung surfactant extract. Proc. Natl. Acad. Sci. USA 2017, 114, E3592–E3601. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhu, P.; Morris, M.D.; Ramamoorthy, A. Solid-State NMR Spectroscopy Provides Atomic-Level Insights Into the Dehydration of Cartilage. J. Phys. Chem. B 2011, 115, 9948–9954. [Google Scholar] [CrossRef] [Green Version]
- Pham, Q.D.; Topgaard, D.; Sparr, E. Tracking solvents in the skin through atomically resolved measurements of molecular mobility in intact stratum corneum. Proc. Natl. Acad. Sci. USA 2017, 114, E112–E121. [Google Scholar] [CrossRef] [Green Version]
- Bjorklund, S.; Andersson, J.M.; Pham, Q.D.; Nowacka, A.; Topgaard, D.; Sparr, E. Stratum corneum molecular mobility in the presence of natural moisturizers. Soft Matter 2014, 10, 4535–4546. [Google Scholar] [CrossRef] [PubMed]
- Corti, M.; Boretta, M.; Cantù, L.; Del Favero, E.; Lesieur, P. Dependence of the form factor of ganglioside micelles on a conformational change with temperature. J. Mol. Struct. 1996, 383, 91–98. [Google Scholar] [CrossRef]
- Hirai, M.; Arai, S.; Takizawa, T.; Yabuki, S.; Nakata, Y. Characteristics of thermotropic phase transition of glycosphingolipid (Ganglioside) aggregates in aqueous solution. Thermochim. Acta 1998, 308, 93–99. [Google Scholar] [CrossRef]
- Ainalem, M.L.; Kristen, N.; Edler, K.J.; Hook, F.; Sparr, E.; Nylander, T. DNA binding to zwitterionic model membranes. Langmuir 2010, 26, 4965–4976. [Google Scholar] [CrossRef] [PubMed]
- Maheux, S.; Frache, G.; Thomann, J.S.; Clément, F.; Penny, C.; Belmonte, T.; Duday, D. Small unilamellar liposomes as a membrane model for cell inactivation by cold atmospheric plasma treatment. J. Phys. D Appl. Phys. 2016, 49, 344001. [Google Scholar] [CrossRef]
- Evans, D.F.; Wennerström, H. The Colloidal Domain: Where Physics, Chemistry, Biology and Technology Meet, 2nd ed.; Wiley-Vch: New York, NY, USA, 1999; Chapter 1. [Google Scholar]
- Lourvanij, K. Partial Dehydration of Glucose to Oxygenated Hydrocarbons in Molecular-Sieving Catalysts. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1995. [Google Scholar]
- Corti, M.; Degiorgio, V.; Ghidoni, R.; Sonnino, S.; Tettamanti, G. Laser-light scattering investigation of the micellar properties of gangliosides. Chem. Phys. Lipids 1980, 26, 225–238. [Google Scholar] [CrossRef]
- Marsh, D. Handbook of Lipid Bilayers; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Rauvala, H. Monomer-micelle transition of the ganglioside GM1 and the hydrolysis by Clostridium perfringens neuraminidase. Eur. J. Biochem. 1979, 97, 555–564. [Google Scholar] [CrossRef]
- Yohe, H.C.; Rosenberg, A. Interaction of triiodide anion with gangliosides in aqueous iodine. Chem. Phys. Lipids 1972, 9, 279–294. [Google Scholar] [CrossRef]
- Hirai, M.; Takizawa, T.; Yabuki, S.; Hayashi, K. Intermicellar interaction of ganglioside aggregates and structural stability on pH variation. J. Chem. Soc. Faraday Trans. 1996, 92, 4533–4540. [Google Scholar] [CrossRef]
- Costigan, S.C.; Booth, P.J.; Templer, R.H. Estimations of Lipid Bilayer Geometry in Fluid Lamellar Phases. Biochim. Biophys. Acta 2000, 1468, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Reed, R.A.; Shipley, G.G. Properties of ganglioside GM1 in phosphatidylcholine bilayer membranes. Biophys. J. 1996, 70, 1363–1372. [Google Scholar] [CrossRef] [Green Version]
- Dave, P.C.; Tiburu, E.K.; Damodaran, K.; Lorigan, G.A. Investigating structural changes in the lipid bilayer upon insertion of the transmembrane domain of the membrane-bound protein phospholamban utilizing 31P and 2H solid-state NMR spectroscopy. Biophys. J. 2004, 86, 1564–1573. [Google Scholar] [CrossRef] [Green Version]
- Leal, C.; Rögnvaldsson, S.; Fossheim, S.; Nilssen, E.A.; Topgaard, D. Dynamic and structural aspects of PEGylated liposomes monitored by NMR. J. Colloid Interface Sci. 2008, 325, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Seelig, J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim. Biophys. Acta 1978, 515, 105–140. [Google Scholar] [CrossRef]
- Tyler, A.I.; Clarke, J.A.; Seddon, J.M.; Law, R.V. Solid state NMR of lipid model membranes. Methods Mol. Biol. (Clifton NJ) 2015, 1232, 227–253. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, H.; Hong, M. Distinguishing Bicontinuous Lipid Cubic Phases from Isotropic Membrane Morphologies Using 31P Solid-State NMR Spectroscopy. J. Phys. Chem. B 2015, 119, 4993–5001. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.S.; Park, S.; Wu, E.L.; Yeom, M.S.; Widmalm, G.; Klauda, J.B.; Im, W. Influence of Ganglioside GM1 Concentration on Lipid Clustering and Membrane Properties and Curvature. Biophys. J. 2016, 111, 1987–1999. [Google Scholar] [CrossRef] [Green Version]
- Carrer, D.C.; Maggio, B. Transduction to self-assembly of molecular geometry and local interactions in mixtures of ceramides and ganglioside GM1. Biochim. Biophys. Acta (Bba) Biomembr. 2001, 1514, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Bedu-Addo, F.K.; Huang, L. Effect of matrix lipid chain length on liposomes containing cholesterol and ganglioside GM1: Implications in drug delivery. J. Pharm. Sci. 1996, 85, 714–719. [Google Scholar] [CrossRef]
- Bunow, M.R.; Bunow, B. Phase behavior of ganglioside-lecithin mixtures. Relation to dispersion of gangliosides in membranes. Biophys. J. 1979, 27, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Cantu, L.; Corti, M.; Sonnino, S.; Tettamanti, G. Evidence for spontaneous segregation phenomena in mixed micelles of gangliosides. Chem. Phys. Lipids 1990, 55, 223–229. [Google Scholar] [CrossRef]
- McIntosh, T.J.; Simon, S.A. Long- and Short-Range Interactions between Phospholipid/Ganglioside GM1 Bilayers. Biochemistry 1994, 33, 10477–10486. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Thompson, T.E.; Barenholz, Y.; Lichtenberg, D. Kinetics of transfer of gangliosides from their micelles to dipalmitoylphosphatidylcholine vesicles. Biochemistry 1983, 22, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Elvang, P.A.; Hinna, A.H.; Brouwers, J.; Hens, B.; Augustijns, P.; Brandl, M. Bile Salt Micelles and Phospholipid Vesicles Present in Simulated and Human Intestinal Fluids: Structural Analysis by Flow Field-Flow Fractionation/Multiangle Laser Light Scattering. J. Pharm. Sci. 2016, 105, 2832–2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, R.; Schmidt, K.H. Structural changes in vesicle membranes and mixed micelles of various lipid compositions after binding of different bile salts. Biochemistry 1988, 27, 8787–8794. [Google Scholar] [CrossRef]
- Johnsson, M.; Edwards, K. Liposomes, Disks, and Spherical Micelles: Aggregate Structure in Mixtures of Gel Phase Phosphatidylcholines and Poly(Ethylene Glycol)-Phospholipids. Biophys. J. 2003, 85, 3839–3847. [Google Scholar] [CrossRef] [Green Version]
- Zetterberg, M.M.; Ahlgren, S.; Agmo Hernandez, V.; Parveen, N.; Edwards, K. Optimization of lipodisk properties by modification of the extent and density of the PEG corona. J. Colloid Interface Sci. 2016, 484, 86–96. [Google Scholar] [CrossRef]
- Galvagnion, C.; Buell, A.K.; Meisl, G.; Michaels, T.C.; Vendruscolo, M.; Knowles, T.P.; Dobson, C.M. Lipid vesicles trigger alpha-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol. 2015, 11, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Pfefferkorn, C.M.; Heinrich, F.; Sodt, A.J.; Maltsev, A.S.; Pastor, R.W.; Lee, J.C. Depth of alpha-synuclein in a bilayer determined by fluorescence, neutron reflectometry, and computation. Biophys. J. 2012, 102, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Stockl, M.; Fischer, P.; Wanker, E.; Herrmann, A. Alpha-synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J. Mol. Biol. 2008, 375, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, D.; Kutluay, E.; Bussell, R.; Browne, G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J. Mol. Biol. 2001, 307, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Jao, C.C.; Hegde, B.G.; Chen, J.; Haworth, I.S.; Langen, R. Structure of membrane-bound α-synuclein from site-directed spin labeling and computational refinement. Proc. Natl. Acad. Sci. USA 2008, 105, 19666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulmer, T.S.; Bax, A.; Cole, N.B.; Nussbaum, R.L. Structure and dynamics of micelle-bound human alpha-synuclein. J. Biol. Chem. 2005, 280, 9595–9603. [Google Scholar] [CrossRef] [Green Version]
- Iyer, A.; Claessens, M. Disruptive membrane interactions of alpha-synuclein aggregates. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 468–482. [Google Scholar] [CrossRef]
- Migdalska-Richards, A.; Schapira, A.H. The relationship between glucocerebrosidase mutations and Parkinson disease. J. Neurochem. 2016, 139, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.I.E.; Katsuse, O.; Yamaguchi, A.; Katsuyama, K.; Aoki, I.; Kosaka, K. Neuronal Accumulation of A- and Β-Synucleins in the Brain of a Gm2 Gangliosidosis Mouse Model. Neuroreport 2013, 14, 551–554. [Google Scholar] [CrossRef]







| Sample | Lipid Molar Composition DOPC:GM1 | Total Lipid Concentration (mM) | ||||
|---|---|---|---|---|---|---|
| Name | cryo-TEM | NMR * | SAXS | DLS | DSC | |
| DOPC | 100:0 | - | 25 | 25 | - | - |
| GM35DOPC65 | 65:35 | 20 | 25 | 25 | - | - |
| GM10DOPC90 | 90:10 | 20 | 25 | 25 | 2 | - |
| GM2.5DOPC97.5 | 97.5: 2.5 | - | 25 | 25 | - | - |
| GM1 | 0:100 | 15 | 15 | 25 & 5 | 3 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojumdar, E.H.; Grey, C.; Sparr, E. Self-Assembly in Ganglioside‒Phospholipid Systems: The Co-Existence of Vesicles, Micelles, and Discs. Int. J. Mol. Sci. 2020, 21, 56. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010056
Mojumdar EH, Grey C, Sparr E. Self-Assembly in Ganglioside‒Phospholipid Systems: The Co-Existence of Vesicles, Micelles, and Discs. International Journal of Molecular Sciences. 2020; 21(1):56. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010056
Chicago/Turabian StyleMojumdar, Enamul Haque, Carl Grey, and Emma Sparr. 2020. "Self-Assembly in Ganglioside‒Phospholipid Systems: The Co-Existence of Vesicles, Micelles, and Discs" International Journal of Molecular Sciences 21, no. 1: 56. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010056