Reduction of Secreted Frizzled-Related Protein 5 Drives Vascular Calcification through Wnt3a-Mediated Rho/ROCK/JNK Signaling in Chronic Kidney Disease
Abstract
:1. Introduction
2. Results
2.1. Expression Profiling of Wnt Signaling Pathway Molecules in an Animal Model of Adenine-Induced CKD with VC
2.2. Effect of CKD on RUNX2, sFRP4, and sFRP5 Expression in Vascular Smooth Muscle Cells
2.3. Effect of sFRP5 on RUNX2 in VSMCs in the CKD Environment
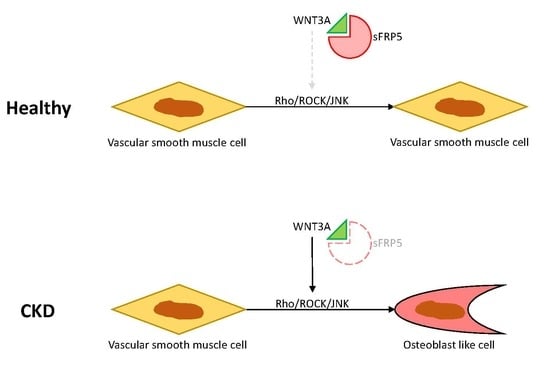
2.4. The Protective Effect of sFRP5 against VSMC Differentiation Is Mediated through the Inhibition of Noncanonical Wnt Signaling
2.5. Effects of Rho/ROCK and the JNK Pathway on sFRP5 Expression in VSMCs in the CKD Environment
2.6. Wnt3a-Enhanced Activation of Noncanonical Wnt Signaling Contributes to Osteoblastic Differentiation of VSMCs
2.7. Expression of RUNX2 and Wnt Signaling–Related Factors in a Rat Model of Adenine-Induced CKD
2.8. Association of the Serum sFRP5 Level with VC in Human Subjects
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Animal Models
4.3. Von Kossa Staining
4.4. Western Blot Analysis
4.5. RNA Profiler PCR Array
4.6. Human Subjects and Measurement of VC
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2009, 113, S1. [Google Scholar]
- Jin, K.; Ban, T.H.; Jung, J.Y.; Kim, A.J.; Kim, Y.; Lee, S.Y.; Yang, D.H.; Choi, B.S.; Oh, K.H.; Kim, J.; et al. Stabilization of serum alkaline phosphatase in hemodialysis patients by implementation of local chronic kidney disease-mineral bone disorder management strategy: A quality improvement study. Kidney Res. Clin. Pract. 2018, 37, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Yoo, K.D.; Kim, H.J.; Koh, J.; Yu, Y.; Kwon, Y.J.; Kim, G.H.; Yoo, T.H.; Lee, J.; Jin, D.C.; et al. Association of serum mineral parameters with mortality in hemodialysis patients: Data from the Korean end-stage renal disease registry. Kidney Res. Clin. Pract. 2018, 37, 266–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naves, M.; Rodriguez-Garcia, M.; Diaz-Lopez, J.B.; Gomez-Alonso, C.; Cannata-Andia, J.B. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos. Int. 2008, 19, 1161–1166. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.; Gomez-Alonso, C.; Naves-Diaz, M.; Diaz-Lopez, J.B.; Diaz-Corte, C.; Cannata-Andia, J.B. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrol. Dial. Transplant. 2009, 24, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Hampson, G.; Edwards, S.; Conroy, S.; Blake, G.M.; Fogelman, I.; Frost, M.L. The relationship between inhibitors of the Wnt signalling pathway (Dickkopf-1(DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone 2013, 56, 42–47. [Google Scholar] [CrossRef]
- Canalis, E. Wnt signalling in osteoporosis: Mechanisms and novel therapeutic approaches. Nat. Rev. Endocrinol. 2013, 9, 575–583. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef]
- McClung, M.R.; Grauer, A.; Boonen, S.; Bolognese, M.A.; Brown, J.P.; Diez-Perez, A.; Langdahl, B.L.; Reginster, J.Y.; Zanchetta, J.R.; Wasserman, S.M.; et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2014, 370, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Glantschnig, H.; Hampton, R.A.; Lu, P.; Zhao, J.Z.; Vitelli, S.; Huang, L.; Haytko, P.; Cusick, T.; Ireland, C.; Jarantow, S.W.; et al. Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J. Biol. Chem. 2010, 285, 40135–40147. [Google Scholar] [CrossRef] [Green Version]
- Tsaousi, A.; Mill, C.; George, S.J. The Wnt pathways in vascular disease: Lessons from vascular development. Curr. Opin. Lipidol. 2011, 22, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Evenepoel, P.; Vervloet, M.G.; Wanner, C.; Ketteler, M.; Marx, N.; Floege, J.; Dekker, F.W.; Brandenburg, V.M. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: Results from the NECOSAD study. Nephrol. Dial. Transplant. 2015, 30, 288–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.S.; Cheng, S.L.; Pingsterhaus, J.M.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J. Clin. Investig. 2005, 115, 1210–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, A.; Radhakrishnan, J.; Wang, H.; Mani, A.; Mani, M.A.; Nelson-Williams, C.; Carew, K.S.; Mane, S.; Najmabadi, H.; Wu, D.; et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 2007, 315, 1278–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarzani, R.; Salvi, F.; Bordicchia, M.; Guerra, F.; Battistoni, I.; Pagliariccio, G.; Carbonari, L.; Dessi-Fulgheri, P.; Rappelli, A. Carotid artery atherosclerosis in hypertensive patients with a functional LDL receptor-related protein 6 gene variant. NMCD 2011, 21, 150–156. [Google Scholar] [CrossRef]
- Xin, H.; Xin, F.; Zhou, S.; Guan, S. The Wnt5a/Ror2 pathway is associated with determination of the differentiation fate of bone marrow mesenchymal stem cells in vascular calcification. Int. J. Mol. Med. 2013, 31, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Guan, S.; Wang, Z.; Xin, F.; Xin, H. Wnt5a is associated with the differentiation of bone marrow mesenchymal stem cells in vascular calcification by connecting with different receptors. Mol. Med. Rep. 2014, 10, 1985–1991. [Google Scholar] [CrossRef]
- Albanese, I.; Yu, B.; Al-Kindi, H.; Barratt, B.; Ott, L.; Al-Refai, M.; de Varennes, B.; Shum-Tim, D.; Cerruti, M.; Gourgas, O.; et al. Role of Noncanonical Wnt Signaling Pathway in Human Aortic Valve Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Dawson, K.; Aflaki, M.; Nattel, S. Role of the Wnt-Frizzled system in cardiac pathophysiology: A rapidly developing, poorly understood area with enormous potential. J. Physiol. 2013, 591, 1409–1432. [Google Scholar] [CrossRef]
- Marinissen, M.J.; Chiariello, M.; Tanos, T.; Bernard, O.; Narumiya, S.; Gutkind, J.S. The Small GTP-Binding Protein RhoA Regulates c-Jun by a ROCK-JNK Signaling Axis. Mol. Cell 2004, 14, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Rankin, S.A.; Sinner, D.; Kenny, A.P.; Krieg, P.A.; Zorn, A.M. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008, 22, 3050–3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, N.; Higuchi, A.; Ohashi, K.; Oshima, Y.; Gokce, N.; Shibata, R.; Akasaki, Y.; Shimono, A.; Walsh, K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 2010, 329, 454–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Sano, S.; Fuster, J.J.; Kikuchi, R.; Shimizu, I.; Ohshima, K.; Katanasaka, Y.; Ouchi, N.; Walsh, K. Secreted frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia-reperfusion injury. J. Biol. Chem. 2015, 291, 2566–2575. [Google Scholar] [CrossRef] [Green Version]
- Claes, K.J.; Viaene, L.; Heye, S.; Meijers, B.; d’Haese, P.; Evenepoel, P. Sclerostin: Another vascular calcification inhibitor? J. Clin. Endocrinol. Metab. 2013, 98, 3221–3228. [Google Scholar] [CrossRef]
- De Mare, A.; Maudsley, S.; Azmi, A.; Hendrickx, J.O.; Opdebeeck, B.; Neven, E.; D’Haese, P.C.; Verhulst, A. Sclerostin as Regulatory Molecule in Vascular Media Calcification and the Bone-Vascular Axis. Toxins 2019, 11, 428. [Google Scholar] [CrossRef] [Green Version]
- Mohler, E.R., 3rd; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone formation and inflammation in cardiac valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef]
- Caira, F.C.; Stock, S.R.; Gleason, T.G.; McGee, E.C.; Huang, J.; Bonow, R.O.; Spelsberg, T.C.; McCarthy, P.M.; Rahimtoola, S.H.; Rajamannan, N.M. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J. Am. Coll. Cardiol. 2006, 47, 1707–1712. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Ginsberg, C.; Seifert, M.; Agapova, O.; Sugatani, T.; Register, T.C.; Freedman, B.I.; Monier-Faugere, M.C.; Malluche, H.; Hruska, K.A. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J. Am. Soc. Nephrol. 2014, 25, 1760–1773. [Google Scholar] [CrossRef] [Green Version]
- Kanbay, M.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Gok, M.; Cetinkaya, H.; Karaman, M.; Unal, H.U.; Oguz, Y.; Sari, S.; et al. Serum sclerostin and adverse outcomes in nondialyzed chronic kidney disease patients. J. Clin. Endocrinol. Metab. 2014, 99, E1854–E1861. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, S.; Dubourg, L.; Carlier, M.C.; Hadj-Aissa, A.; Fouque, D. The relation between renal function and serum sclerostin in adult patients with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.R.; Olauson, H.; Witasp, A.; Haarhaus, M.; Brandenburg, V.; Wernerson, A.; Lindholm, B.; Soderberg, M.; Wennberg, L.; Nordfors, L.; et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int. 2015, 88, 1356–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Cai, Y.; Luo, L.M.; Wang, R. Expression of Wnt and NCX1 and its correlation with cardiomyocyte apoptosis in mouse with myocardial hypertrophy. Asian Pac. J. Trop. Med. 2015, 8, 930–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.K.; Kang, Y.M.; Lee, S.E.; Lee, Y.; Seol, S.M.; Lee, W.J.; Park, J.Y.; Jung, C.H. Effect of SFRP5 (Secreted Frizzled-Related Protein 5) on the WNT5A (Wingless-Type Family Member 5A)-Induced Endothelial Dysfunction and Its Relevance With Arterial Stiffness in Human Subjects. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rios, J.; Esteve, P.; Ruiz, J.M.; Bovolenta, P. The Netrin-related domain of Sfrp1 interacts with Wnt ligands and antagonizes their activity in the anterior neural plate. Neural. Dev. 2008, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.B.; Chen, X.D.; Zhou, X.Y.; Zhu, Q. The Wnt antagonist and secreted frizzled-related protein 5: Implications on lipid metabolism, inflammation, and type 2 diabetes mellitus. Biosci. Rep. 2018, 38, BSR20180011. [Google Scholar] [CrossRef]
- Carstensen-Kirberg, M.; Kannenberg, J.M.; Huth, C.; Meisinger, C.; Koenig, W.; Heier, M.; Peters, A.; Rathmann, W.; Roden, M.; Herder, C.; et al. Inverse associations between serum levels of secreted frizzled-related protein-5 (SFRP5) and multiple cardiometabolic risk factors: KORA F4 study. Cardiovasc. Diabetol. 2017, 16, 109. [Google Scholar] [CrossRef]
- Miyoshi, T.; Doi, M.; Usui, S.; Iwamoto, M.; Kajiya, M.; Takeda, K.; Nosaka, K.; Nakayama, R.; Okawa, K.; Takagi, W.; et al. Low serum level of secreted frizzled-related protein 5, an anti-inflammatory adipokine, is associated with coronary artery disease. Atherosclerosis 2014, 233, 454–459. [Google Scholar] [CrossRef]
- Deng, D.; Diao, Z.; Han, X.; Liu, W. Secreted Frizzled-Related Protein 5 Attenuates High Phosphate-Induced Calcification in Vascular Smooth Muscle Cells by Inhibiting the Wnt/ss-Catenin Pathway. Calcif. Tissue Int. 2016, 99, 66–75. [Google Scholar] [CrossRef]
- Chatani, N.; Kamada, Y.; Kizu, T.; Ogura, S.; Furuta, K.; Egawa, M.; Hamano, M.; Ezaki, H.; Kiso, S.; Shimono, A.; et al. Secreted frizzled-related protein 5 (Sfrp5) decreases hepatic stellate cell activation and liver fibrosis. Liver Int. 2015, 35, 2017–2026. [Google Scholar] [CrossRef]
- Mori, H.; Prestwich, T.C.; Reid, M.A.; Longo, K.A.; Gerin, I.; Cawthorn, W.P.; Susulic, V.S.; Krishnan, V.; Greenfield, A.; Macdougald, O.A. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J. Clin. Investig. 2012, 122, 2405–2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobune, M.; Chiba, H.; Kato, J.; Kato, K.; Nakamura, K.; Kawano, Y.; Takada, K.; Takimoto, R.; Takayama, T.; Hamada, H.; et al. Wnt3/RhoA/ROCK signaling pathway is involved in adhesion-mediated drug resistance of multiple myeloma in an autocrine mechanism. Mol. Cancer Ther. 2007, 6, 1774–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalesso, G.; Sherwood, J.; Bertrand, J.; Pap, T.; Ramachandran, M.; De Bari, C.; Pitzalis, C.; Dell’accio, F. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J. Cell Biol. 2011, 193, 551–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topol, L.; Jiang, X.; Choi, H.; Garrett-Beal, L.; Carolan, P.J.; Yang, Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 2003, 162, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Yamamoto, H.; Sakane, H.; Koyama, H.; Kikuchi, A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010, 29, 41–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berndt, T.; Craig, T.A.; Bowe, A.E.; Vassiliadis, J.; Reczek, D.; Finnegan, R.; Jan De Beur, S.M.; Schiavi, S.C.; Kumar, R. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J. Clin. Investig. 2003, 112, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.T.; Esumi, N.; Moore, K.; Li, Y.; Zhang, S.; Chew, C.; Goodman, B.; Rattner, A.; Moody, S.; Stetten, G.; et al. Cloning and characterization of a secreted frizzled-related protein that is expressed by the retinal pigment epithelium. Hum. Mol. Genet. 1999, 8, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Guo, B.; Yan, J.; Yang, R.; Chang, L.; Wang, Y.; Miao, C.; Liu, S.; Zhang, H.; Li, Y. Angiotensin II increases secreted frizzled-related protein 5 (sFRP5) expression through AT1 receptor/Rho/ROCK1/JNK signaling in cardiomyocytes. Mol. Cell. Biochem. 2015, 408, 215–222. [Google Scholar] [CrossRef]
- Hu, W.; Li, L.; Yang, M.; Luo, X.; Ran, W.; Liu, D.; Xiong, Z.; Liu, H.; Yang, G. Circulating Sfrp5 is a signature of obesity-related metabolic disorders and is regulated by glucose and liraglutide in humans. J. Clin. Endocrinol. Metab. 2013, 98, 290–298. [Google Scholar] [CrossRef]
- Almario, R.U.; Karakas, S.E. Roles of circulating WNT-signaling proteins and WNT-inhibitors in human adiposity, insulin resistance, insulin secretion, and inflammation. Horm. Metab. Res. 2015, 47, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zhang, D.; Chen, B. Declined plasma sfrp5 concentration in patients with type 2 diabetes and latent autoimmune diabetes in adults. Pak. J. Med. Sci. 2015, 31, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Derda, A.A.; Woo, C.C.; Wongsurawat, T.; Richards, M.; Lee, C.N.; Kofidis, T.; Kuznetsov, V.A.; Sorokin, V.A. Gene expression profile analysis of aortic vascular smooth muscle cells reveals upregulation of cadherin genes in myocardial infarction patients. Physiol. Genom. 2018, 50, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Wongsurawat, T.; Woo, C.C.; Giannakakis, A.; Lin, X.Y.; Cheow, E.S.H.; Lee, C.N.; Richards, M.; Sze, S.K.; Nookaew, I.; Kuznetsov, V.A.; et al. Transcriptome alterations of vascular smooth muscle cells in aortic wall of myocardial infarction patients. Data Brief 2018, 17, 1112–1135. [Google Scholar] [CrossRef] [PubMed]
- Wongsurawat, T.; Woo, C.C.; Giannakakis, A.; Lin, X.Y.; Cheow, E.S.H.; Lee, C.N.; Richards, M.; Sze, S.K.; Nookaew, I.; Kuznetsov, V.A.; et al. Distinctive molecular signature and activated signaling pathways in aortic smooth muscle cells of patients with myocardial infarction. Atherosclerosis 2018, 271, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Price, P.A.; Roublick, A.M.; Williamson, M.K. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int. 2006, 70, 1577–1583. [Google Scholar] [CrossRef] [Green Version]
- Shobeiri, N.; Adams, M.A.; Holden, R.M. Vascular calcification in animal models of CKD: A review. Am. J. Nephrol. 2010, 31, 471–481. [Google Scholar] [CrossRef]
- Neven, E.; D’Haese, P.C. Vascular calcification in chronic renal failure: What have we learned from animal studies? Circ. Res. 2011, 108, 249–264. [Google Scholar] [CrossRef]
- Henley, C.; Colloton, M.; Cattley, R.C.; Shatzen, E.; Towler, D.A.; Lacey, D.; Martin, D. 1,25-Dihydroxyvitamin D3 but not cinacalcet HCl (Sensipar/Mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol. Dial. Transplant. 2005, 20, 1370–1377. [Google Scholar] [CrossRef] [Green Version]
- Henley, C.; Davis, J.; Miller, G.; Shatzen, E.; Cattley, R.; Li, X.; Martin, D.; Yao, W.; Lane, N.; Shalhoub, V. The calcimimetic AMG 641 abrogates parathyroid hyperplasia, bone and vascular calcification abnormalities in uremic rats. Eur. J. Pharmacol. 2009, 616, 306–313. [Google Scholar] [CrossRef]
- Kauppila, L.I.; Polak, J.F.; Cupples, L.A.; Hannan, M.T.; Kiel, D.P.; Wilson, P.W. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis 1997, 132, 245–250. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Lee, O.H.; Kim, M.J.; Joo, W.C.; Lee, S.Y.; Kim, M.-J.; Song, J.H.; Lee, S.W. The association between mortality and abdominal aortic calcification and relation between its progression and serum calcium concentration in chronic hemodialysis patients. Kidney Res. Clin. Pract. 2014, 33, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.J.; Kim, H.; Kim, A.J.; Ro, H.; Chang, J.H.; Lee, H.H.; Chung, W.; Jun, H.-S.; Jung, J.Y. Reduction of Secreted Frizzled-Related Protein 5 Drives Vascular Calcification through Wnt3a-Mediated Rho/ROCK/JNK Signaling in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 3539. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103539
Oh YJ, Kim H, Kim AJ, Ro H, Chang JH, Lee HH, Chung W, Jun H-S, Jung JY. Reduction of Secreted Frizzled-Related Protein 5 Drives Vascular Calcification through Wnt3a-Mediated Rho/ROCK/JNK Signaling in Chronic Kidney Disease. International Journal of Molecular Sciences. 2020; 21(10):3539. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103539
Chicago/Turabian StyleOh, Yun Jung, Hyunsook Kim, Ae Jin Kim, Han Ro, Jae Hyun Chang, Hyun Hee Lee, Wookyung Chung, Hee-Sook Jun, and Ji Yong Jung. 2020. "Reduction of Secreted Frizzled-Related Protein 5 Drives Vascular Calcification through Wnt3a-Mediated Rho/ROCK/JNK Signaling in Chronic Kidney Disease" International Journal of Molecular Sciences 21, no. 10: 3539. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103539