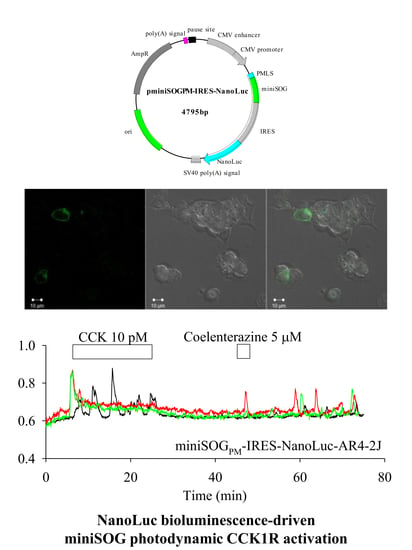
NanoLuc Bioluminescence-Driven Photodynamic Activation of Cholecystokinin 1 Receptor with Genetically-Encoded Protein Photosensitizer MiniSOG
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. AR4-2J and E. coli Cell Culture
4.3. Vector Constructs
4.4. Transduction of AR4-2J Cells
4.5. miniSOG Fluorescence Imaging
4.6. Photodynamic Action
4.7. Calcium Measurements
4.8. Data Presentation and Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CCK1R | Cholecystokinin 1 receptors |
| CNS | Central nervous system |
| GdPLMB | Gadolinium porphyrin-like macrocycle B |
| GEPP | Genetically-encoded protein photosensitizer |
| GPCR | G protein-coupled receptor |
| IRES | Internal ribosome entry site |
| miniSOG | mini singlet oxygen generator |
| LED | Light-emitting diode |
| 1O2 | Singlet oxygen |
| PM | Plasma membrane |
| SALPC | Sulphonated aluminium phthlaocyanine |
Aix A
| NanoLuc [42] | miniSOG [35] | ||||
|---|---|---|---|---|---|
| Peak | 478 nm | 100% | 478 nm | 77% | |
| 448 nm | 68% | Peak | 448 nm | 100% | |
| Light Sources | Power Density | Duration (min) | References |
|---|---|---|---|
| Halogen cold light (white) | 87 mW · cm−2 | 5 | [33] |
| Blue LED (450 nm) | 85 mW · cm−2 | 1.5 | This work |
| NanoLuc + coelenterazine | 5 μM (coelenterazine) | 3 | This work |
References
- Guo, H.Y.; Cui, Z.J. Extracellular histones activate plasma membrane Toll-like receptor 9 to trigger calcium oscillations in rat pancreatic acinar tumor cell AR4-2J. Cells 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.S.; Cui, Z.J. Pancreatic stellate cells serve as a brake mechanism on pancreatic acinar cell calcium signaling modulated by methionine sulfoxide reductase expression. Cells 2019, 8, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, L.D.; Beart, P.M. Histochemistry in rat brain and spinal cord with an antibody directed at the cholecystokinin A receptor. Neurosci. Lett. 1997, 225, 97–100. [Google Scholar] [CrossRef]
- Mercer, L.D.; Beart, P.M. Immunolocalization of CCK1R in rat brain using a new anti-peptide antibody. Neurosci. Lett. 2004, 359, 109–113. [Google Scholar] [CrossRef]
- Honda, T.; Wada, E.; Battey, J.F.; Wank, S.A. Differential gene expression of CCKA and CCKB receptors in the rat brain. Mol. Cell Neurosci. 1993, 4, 143–154. [Google Scholar] [CrossRef]
- Nishimura, S.; Bilgüvar, K.; Ishigame, K.; Sestan, N.; Günel, M.; Louvi, A. Functional synergy between cholecystokinin receptors CCKAR and CCKBR in mammalian brain development. PLoS ONE 2015, 10, e0124295. [Google Scholar] [CrossRef] [Green Version]
- Broberger, C.; Holmberg, K.; Shi, T.J.; Dockray, G.; Hökfelt, T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res. 2001, 903, 128–140. [Google Scholar] [CrossRef]
- Glatzle, J.; Wang, Y.; Adelson, D.W.; Kalogeris, T.J.; Zittel, T.T.; Tso, P.; Wei, J.Y.; Raybould, H.E. Chylomicron components activate duodenal vagal afferents via a cholecystokinin A receptor-mediated pathway to inhibit gastric motor function in the rat. J. Physiol. 2003, 550, 657–664. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Zhou, S.; Owyang, C. Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: Implications for leptin as a regulator of short-term satiety. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G217–G227. [Google Scholar] [CrossRef] [Green Version]
- Mussa, B.M.; Sartor, D.M.; Verberne, A.J. Activation of cholecystokinin (CCK 1) and serotonin (5-HT 3) receptors increases the discharge of pancreatic vagal afferents. Eur. J. Pharmacol. 2008, 601, 198–206. [Google Scholar] [CrossRef]
- Patterson, L.M.; Zheng, H.; Ward, S.M.; Berthoud, H.R. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 2001, 305, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.M.; Zheng, H.; Berthoud, H.R. Vagal afferents innervating the gastrointestinal tract and CCKA-receptor immunoreactivity. Anat. Rec. 2002, 266, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, N.; Yamanaka, A.; Ichiki, K.; Muraki, Y.; Kilduff, T.S.; Yagami, K.; Takahashi, S.; Goto, K.; Sakurai, T. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J. Neurosci. 2005, 25, 7459–7469. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gayo, M.; Garrido, M.M.; Fuentes, J.A. Inhibition of the hypothalamic-pituitary-adrenal axis in food-deprived rats by a CCK-A receptor antagonist. Br. J. Pharmacol. 2000, 129, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Onaka, T.; Kawasaki, M.; Chen, L.; Mera, T.; Soya, A.; Saito, T.; Fujihara, H.; Sei, H.; Morita, Y.; et al. Effects of cholecystokinin (CCK)-8 on hypothalamicoxytocin-secreting neurons in rats lacking CCK-A receptor. Auton. Neurosci. 2005, 121, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Akiyoshi, J.; Kiyota, A.; Katsuragi, S.; Tsutsumi, T.; Isogawa, K.; Nagayama, H. Increased anxiety behavior in OLETF rats without cholecystokinin—A receptor. Brain Res. Bull. 2000, 53, 789–792. [Google Scholar] [CrossRef]
- Wen, D.; Sun, D.; Zang, G.; Hao, L.; Liu, X.; Yu, F.; Ma, C.; Cong, B. Cholecystokinin octapeptide induces endogenous opioid-dependent anxiolytic effects in morphine-withdrawal rats. Neuroscience 2014, 277, 14–25. [Google Scholar] [CrossRef]
- Zhu, G.; Yan, J.; Smith, W.W.; Moran, T.H.; Bi, S. Roles of dorsomedial hypothalamic cholecystokinin signaling in the controls of meal patterns and glucose homeostasis. Physiol. Behav. 2012, 105, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; Vermeulen, R.; Hokfelt, T.; Horne, M.K.; Stanic, D. Female mice lacking cholecystokinin 1 receptors have compromised neurogenesis, and fewer dopaminergic cells in the olfactory bulb. Front. Cell Neurosci. 2013, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, H.; Akiyoshi, J.; Kai, K.; Ishii, N.; Kodama, K.; Tsutsumi, T.; Isogawa, K.; Nagayama, H. Spatial memory impairment in OLETF rats without cholecystokinin-A receptor. Neuropeptides 2003, 37, 271–276. [Google Scholar] [CrossRef]
- Suzuki, S.; Takiguchi, S.; Sato, N.; Kanai, S.; Kawanami, T.; Yoshida, Y.; Miyasaka, K.; Takata, Y.; Funakoshi, A.; Noda, T. Importance of CCK-A receptor for gallbladder contraction and pancreatic secretion: A study in CCK-A receptor knockout mice. Jpn. J. Physiol. 2001, 51, 585–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takiguchi, S.; Suzuki, S.; Sato, Y.; Kanai, S.; Miyasaka, K.; Jimi, A.; Shinozaki, H.; Takata, Y.; Funakoshi, A.; et al. Role of CCK-A receptor for pancreatic function in mice: A study in CCK-A receptor knockout mice. Pancreas 2002, 24, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Malendowicz, L.K.; Nowak, M.; Gottardo, L.; Tortorella, C.; Majchrzak, M.; Nussdorfer, G.G. Cholecystokinin stimulates aldosterone secretion from dispersed rat zona glomerulosa cells, acting through cholecystokinin receptors 1 and 2 coupled with the adenylate cyclase-dependent cascade. Endocrinology 2001, 142, 4251–4255. [Google Scholar] [CrossRef] [PubMed]
- Whited, K.L.; Thao, D.; Lloyd, K.C.; Kopin, A.S.; Raybould, H.E. Targeted disruption of the murine CCK1 receptor gene reduces intestinal lipid-induced feedback inhibition of gastric function. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G156–G162. [Google Scholar] [CrossRef] [Green Version]
- Boeckxstaens, G.E.; Hirsch, D.P.; Fakhry, N.; Holloway, R.H.; D’Amato, M.; Tytgat, G.N. Involvement of cholecystokinin A receptors in transient lower esophageal sphincter relaxations triggered by gastric distension. Am. J. Gastroenterol. 1998, 93, 1823–1828. [Google Scholar] [CrossRef]
- Varga, G.; Bálint, A.; Burghardt, B.; D’Amato, M. Involvement of endogenous CCK and CCK1 receptors in colonic motor function. Br. J. Pharmacol. 2004, 141, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.A. Cholecystokinin (CCK) regulation of pancreatic acinar cells: Physiological actions and signal transduction mechanisms. Compr. Physiol. 2019, 9, 535–564. [Google Scholar] [CrossRef]
- Liang, H.Y.; Song, Z.M.; Cui, Z.J. Lasting inhibition of receptor-mediated calcium oscillations in pancreatic acini by neutrophil respiratory burst—A novel mechanism for secretory blockade in acute pancreatitis? Biochem. Biophys. Res. Commun. 2013, 437, 361–367. [Google Scholar] [CrossRef]
- Cui, Z.J.; Kanno, T. Photodynamic triggering of calcium oscillation in the isolated rat pancreatic acini. J. Physiol. 1997, 504, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.J.; Habara, Y.; Wang, D.Y.; Kanno, T. A novel aspect of photodynamic action: Induction of recurrent spikes in cytosolic calcium concentration. Photochem. Photobiol. 1997, 65, 382–386. [Google Scholar] [CrossRef]
- An, Y.P.; Xiao, R.; Cui, H.; Cui, Z.J. Selective activation by photodynamic action of cholecystokinin receptor in the freshly isolated rat pancreatic acini. Br. J. Pharmacol. 2003, 139, 872–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.N.; Li, Y.; Cui, Z.J. Photodynamic physiology—Photonanomanipulations in cellular physiology with protein photosensitisers. Front. Physiol. 2017, 8, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.N.; Li, Y.; Cui, Z.J. Cholecystokinin 1 receptor-a unique G protein-coupled receptor activated by singlet oxygen (GPCR-ABSO). Front. Physiol. 2018, 9, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, X.; Lev-Ram, V.; Deerinck, T.J.; Qi, Y.; Ramko, E.B.; Davidson, M.W.; Jin, Y.; Ellisman, M.H.; Tsien, R.Y. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011, e1001041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimenta, F.M.; Jensen, R.L.; Breitenbach, T.; Etzerodt, M.; Ogilby, P.R. Oxygen-dependent photochemistry and photophysics of “miniSOG,” a protein-encased flavin. Photochem. Photobiol. 2013, 89, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Torra, J.; Lafaye, C.; Signor, L.; Aumonier, S.; Flors, C.; Shu, X.; Nonell, S.; Gotthard, G.; Royant, A. Tailing miniSOG: Structural bases of the complex photophysics of a flavin-binding singlet oxygen photosensitizing protein. Sci. Rep. 2019, 9, 2428. [Google Scholar] [CrossRef]
- Rodríguez-Pulido, A.; Cortajarena, A.L.; Torra, J.; Ruiz-González, R.; Nonell, S.; Flors, C. Assessing the potential of photosensitizing flavoproteins as tags for correlative microscopy. Chem. Commun. 2016, 52, 8405–8408. [Google Scholar] [CrossRef] [Green Version]
- Endres, S.; Wingen, M.; Torra, J.; Ruiz-Gonzalez, R.; Polen, T.; Bosio, G.; Bitzenhofer, N.L.; Hilgers, F.; Gensch, T.; Nonell, S.; et al. An optogenetic toolbox of LOV-based photosensitizers for light-driven killing of bacteria. Sci. Rep. 2018, 8, 15021. [Google Scholar] [CrossRef] [Green Version]
- Westberg, M.; Bregnhøj, M.; Etzerodt, M.; Ogilby, P.R. No photon wasted: An efficient and selective singlet oxygen photosensitizing protein. J. Phys. Chem. B 2017, 121, 9366–9371. [Google Scholar] [CrossRef]
- Westberg, M.; Etzerodt, M.; Ogilby, P.R. Rational design of genetically encoded singlet oxygen photosensitizing proteins. Curr. Opin. Struct. Biol. 2019, 57, 56–62. [Google Scholar] [CrossRef]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Shakhmin, A.; Hall, M.P.; Machleidt, T.; Walker, J.R.; Wood, K.V.; Kirkland, T.A. Coelenterazine analogues emit red-shifted bioluminescence with NanoLuc. Org. Biomol. Chem. 2017, 15, 8559–8567. [Google Scholar] [CrossRef] [PubMed]
- Machleidt, T.; Woodroofe, C.C.; Schwinn, M.K.; Méndez, J.; Robers, M.B.; Zimmerman, K.; Otto, P.; Daniels, D.L.; Kirkland, T.A.; Wood, K.V. NanoBRET—A novel BRET platform for the analysis of protein-protein interactions. ACS Chem. Biol. 2015, 10, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Lackner, D.H.; Carré, A.; Guzzardo, P.M.; Banning, C.; Mangena, R.; Henley, T.; Oberndorfer, S.; Gapp, B.V.; Nijman, S.M.; Brummelkamp, T.R.; et al. A generic strategy for CRISPR-Cas9-mediated gene tagging. Nat. Commun. 2015, 6, 10237. [Google Scholar] [CrossRef]
- Yasuzaki, Y.; Yamada, Y.; Ishikawa, T.; Harashima, H. Validation of mitochondrial gene delivery in liver and skeletal muscle via hydrodynamic injection using an artificial mitochondrial reporter DNA vector. Mol. Pharm. 2015, 12, 4311–4320. [Google Scholar] [CrossRef]
- Stacer, A.C.; Nyati, S.; Moudgil, P.; Iyengar, R.; Luker, K.E.; Rehemtulla, A.; Luker, G.D. NanoLuc reporter for dual luciferase imaging in living animals. Mol. Imaging 2013, 12, 1–13. [Google Scholar] [CrossRef]
- Germain-Genevois, C.; Garandeau, O.; Couillaud, F. Detection of brain tumors and systemic metastases using NanoLuc and Fluc for dual reporter imaging. Mol. Imaging Biol. 2016, 18, 62–69. [Google Scholar] [CrossRef]
- Shramova, E.I.; Proshkina, G.M.; Chumakov, S.P.; Khodarovich, Y.M.; Deyev, S.M. Flavoprotein miniSOG cytotoxisity can be induced by bioluminescence resonance energy transfer. Acta Nat. 2016, 8, 118–123. [Google Scholar] [CrossRef]
- Shramova, E.I.; Proshkina, G.M.; Deyev, S.M.; Petrov, R.V. Flavoprotein miniSOG BRET-induced cytotoxicity depends on its intracellular localization. Dokl. Biochem. Biophys. 2017, 474, 228–230. [Google Scholar] [CrossRef]
- Proshkina, G.M.; Shramova, E.I.; Shilova, O.N.; Ryabova, A.V.; Deyev, S.M. Phototoxicity of flavoprotein miniSOG induced by bioluminescence resonance energy transfer in genetically encoded system NanoLuc-miniSOG is comparable with its LED-excited phototoxicity. J. Photochem. Photobiol. B Biol. 2018, 188, 107–115. [Google Scholar] [CrossRef]
- Shramova, E.I.; Proshkina, G.M.; Deyev, S.M.; Petrov, R.V. Death mechanism of breast adenocarcinoma cells caused by BRET-induced cytotoxicity of miniSOG depends on the intracellular localization of the NanoLuc-miniSOG fusion protein. Dokl. Biochem. Biophys. 2018, 482, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.J.; Liang, H.Y.; Jin, W.J.; Cui, Z.J. Substance P conjugated to CdTe quantum dot triggers cytosolic calcium oscillations and induces QD internalization in the pancreatic carcinoma cell line AR4-2J. Anal. Bioanal. Chem. 2011, 400, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.F.; Cui, Z.J. The anti-botulism triterpenoid toosendanin elicits calcium increase and exocytosis in rat sensory neurons. Cell Mol. Neurobiol. 2011, 31, 1151–1162. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cui, Z.J. NanoLuc Bioluminescence-Driven Photodynamic Activation of Cholecystokinin 1 Receptor with Genetically-Encoded Protein Photosensitizer MiniSOG. Int. J. Mol. Sci. 2020, 21, 3763. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21113763
Li Y, Cui ZJ. NanoLuc Bioluminescence-Driven Photodynamic Activation of Cholecystokinin 1 Receptor with Genetically-Encoded Protein Photosensitizer MiniSOG. International Journal of Molecular Sciences. 2020; 21(11):3763. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21113763
Chicago/Turabian StyleLi, Yuan, and Zong Jie Cui. 2020. "NanoLuc Bioluminescence-Driven Photodynamic Activation of Cholecystokinin 1 Receptor with Genetically-Encoded Protein Photosensitizer MiniSOG" International Journal of Molecular Sciences 21, no. 11: 3763. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21113763