Bergenin Reduces Experimental Painful Diabetic Neuropathy by Restoring Redox and Immune Homeostasis in the Nervous System
Abstract
:1. Introduction
2. Results
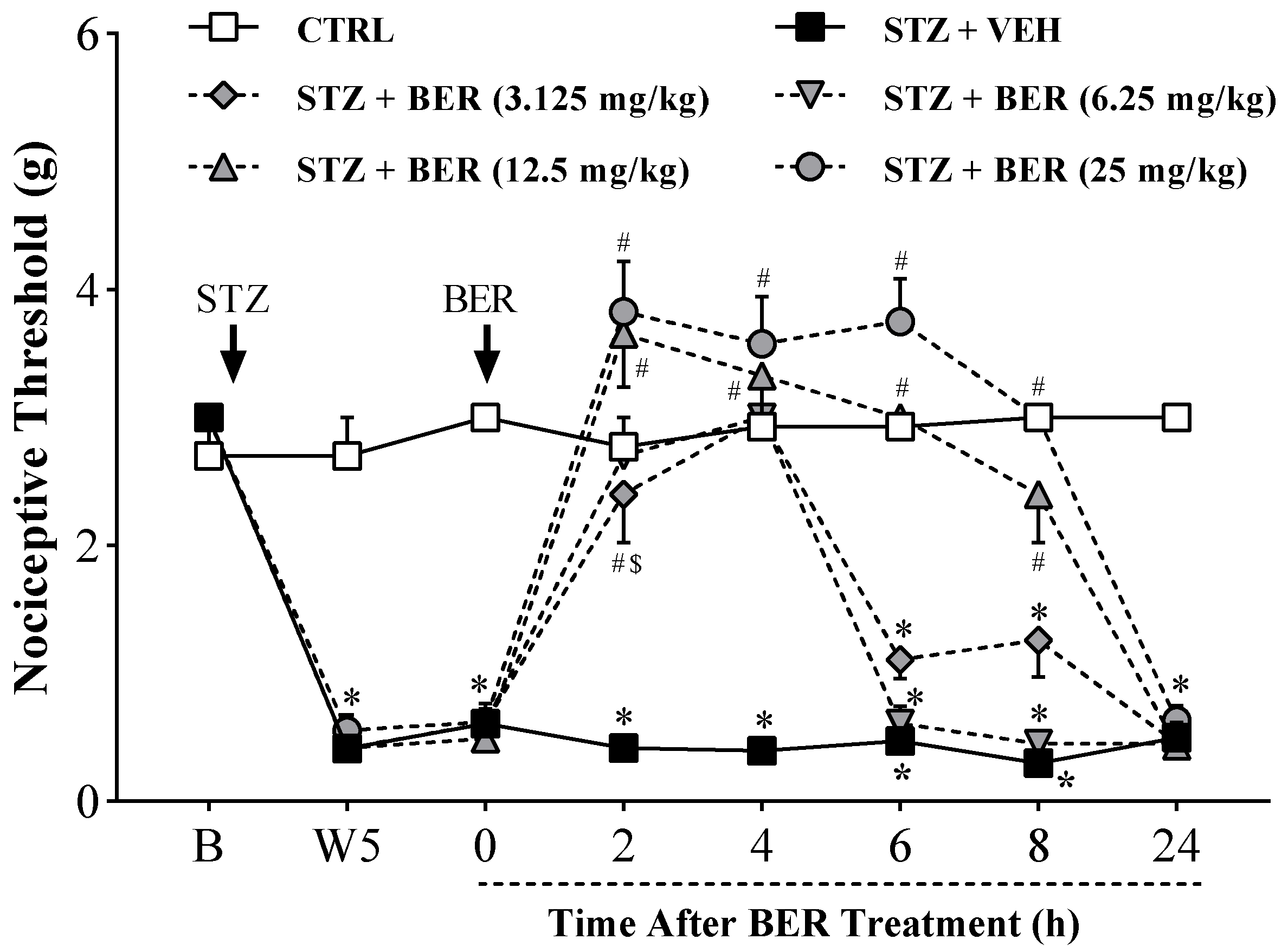
2.1. Acute Bergenin Treatment Ameliorates Established Painful Diabetic Neuropathy
2.2. Multiple Doses of Bergenin Revert Behavioral Painful Diabetic Neuropathy
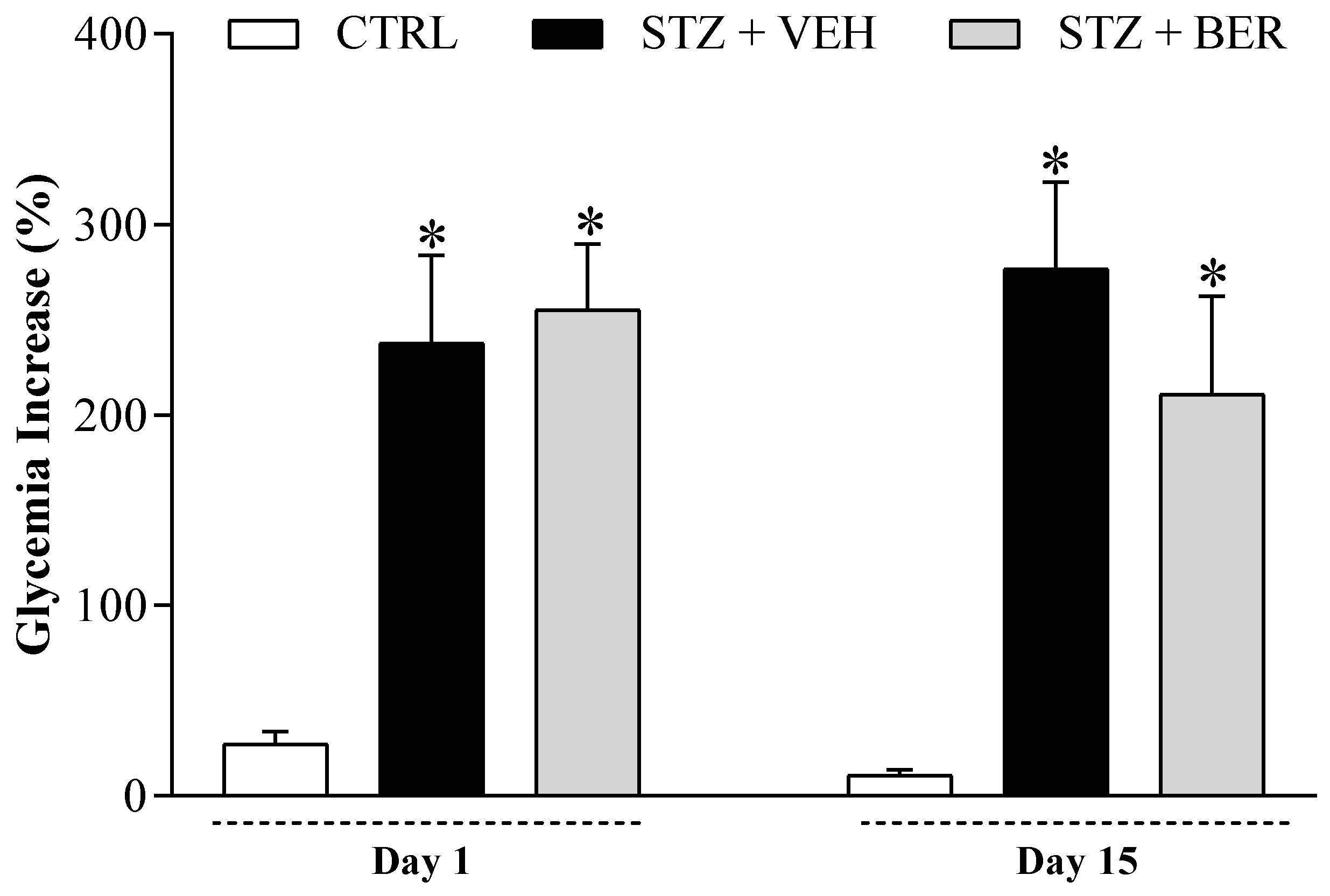
2.3. Bergenin Treatment Does Not Reduce Glycemia in Diabetic Mice
2.4. Bergenin Reduces NO Production in Vitro
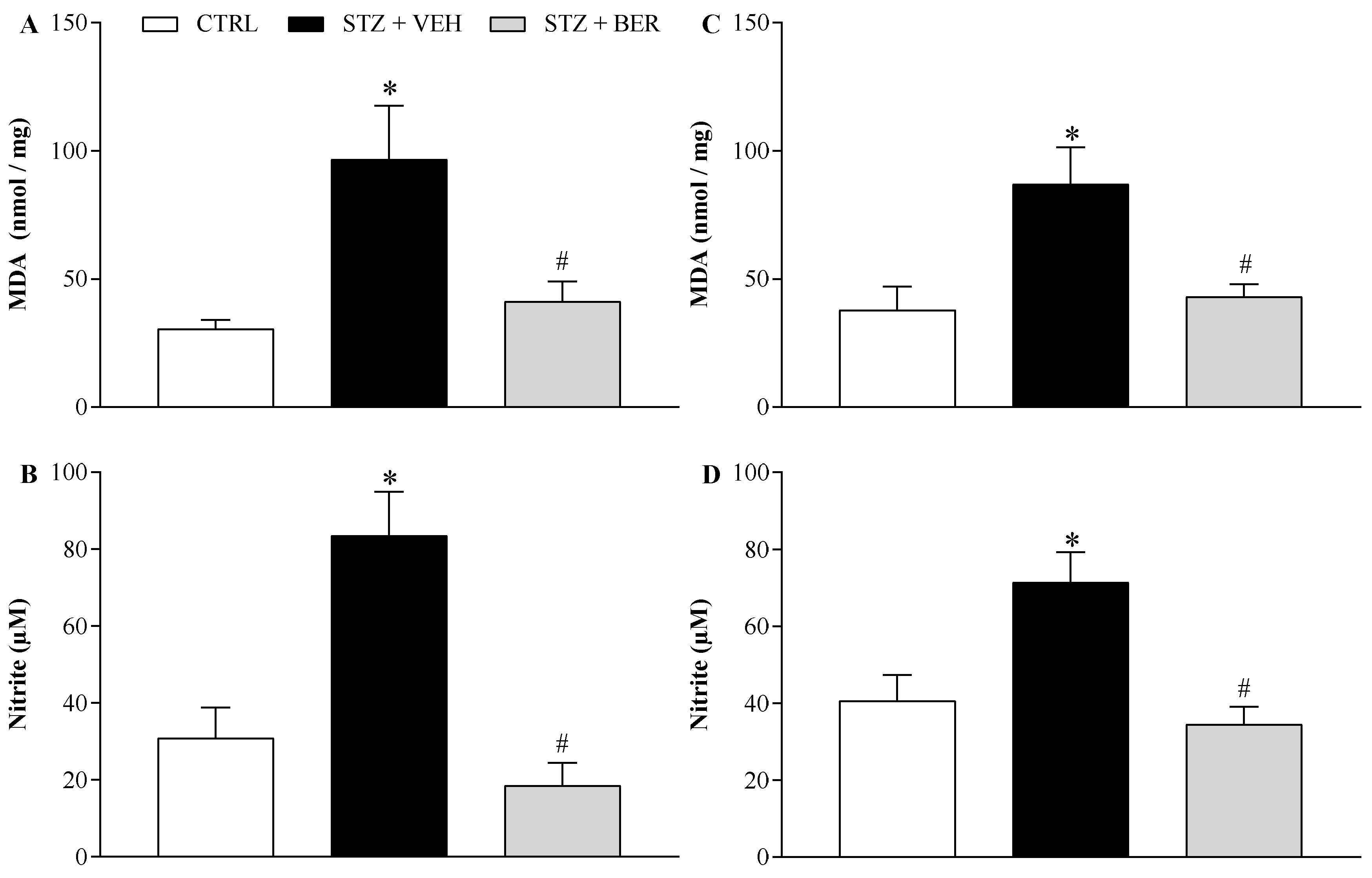
2.5. Bergenin Reduces Markers of Oxidative Stress in Nervous System of Neuropathic Mice
2.6. Bergenin Modifies the Antioxidant Profile in the Nervous System of Mice with Painful DN
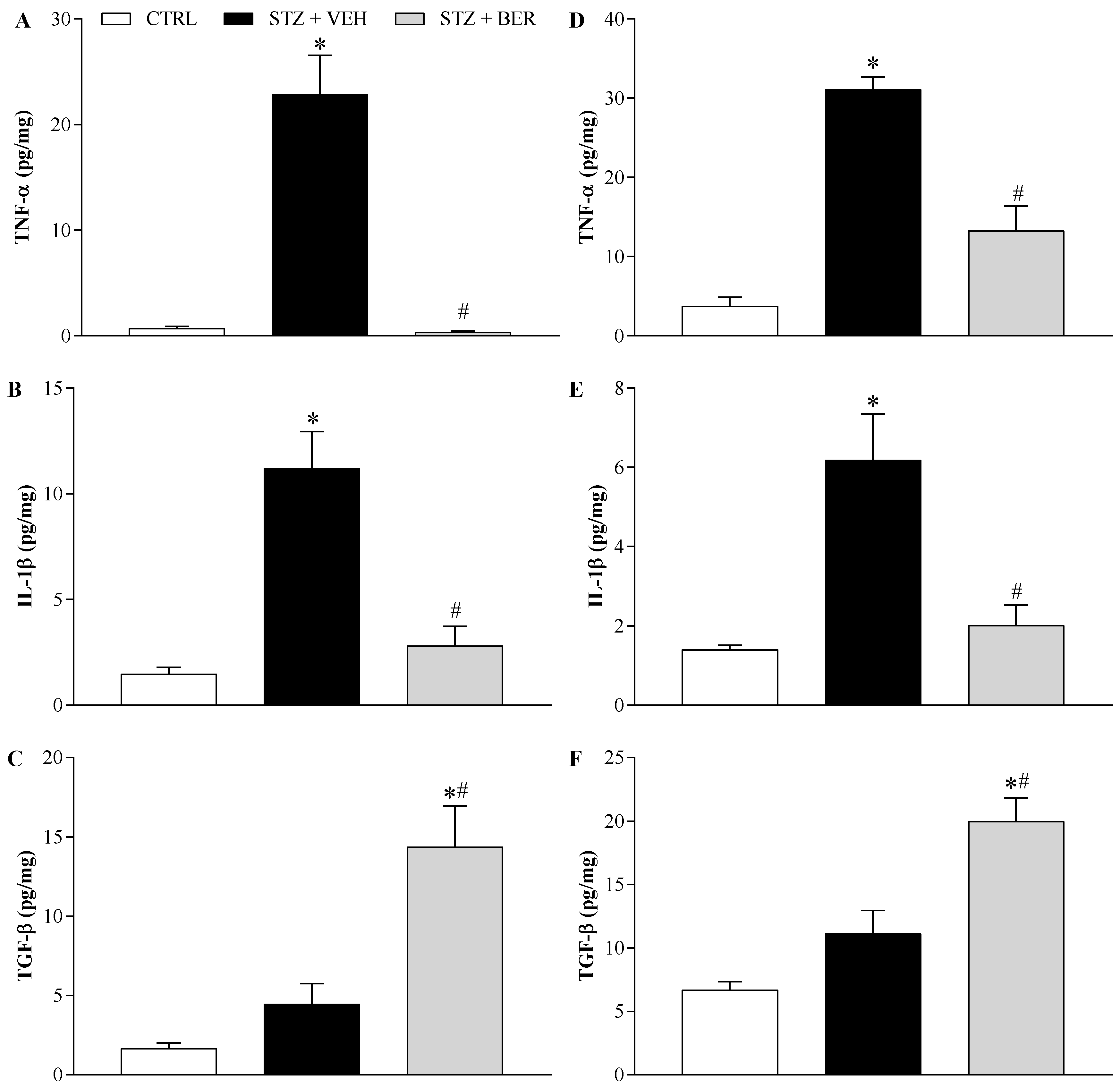
2.7. Bergenin Modulates Pro- and Anti-Inflammatory Cytokines Production in the Nervous System
3. Discussion
4. Materials and Methods
4.1. Animals
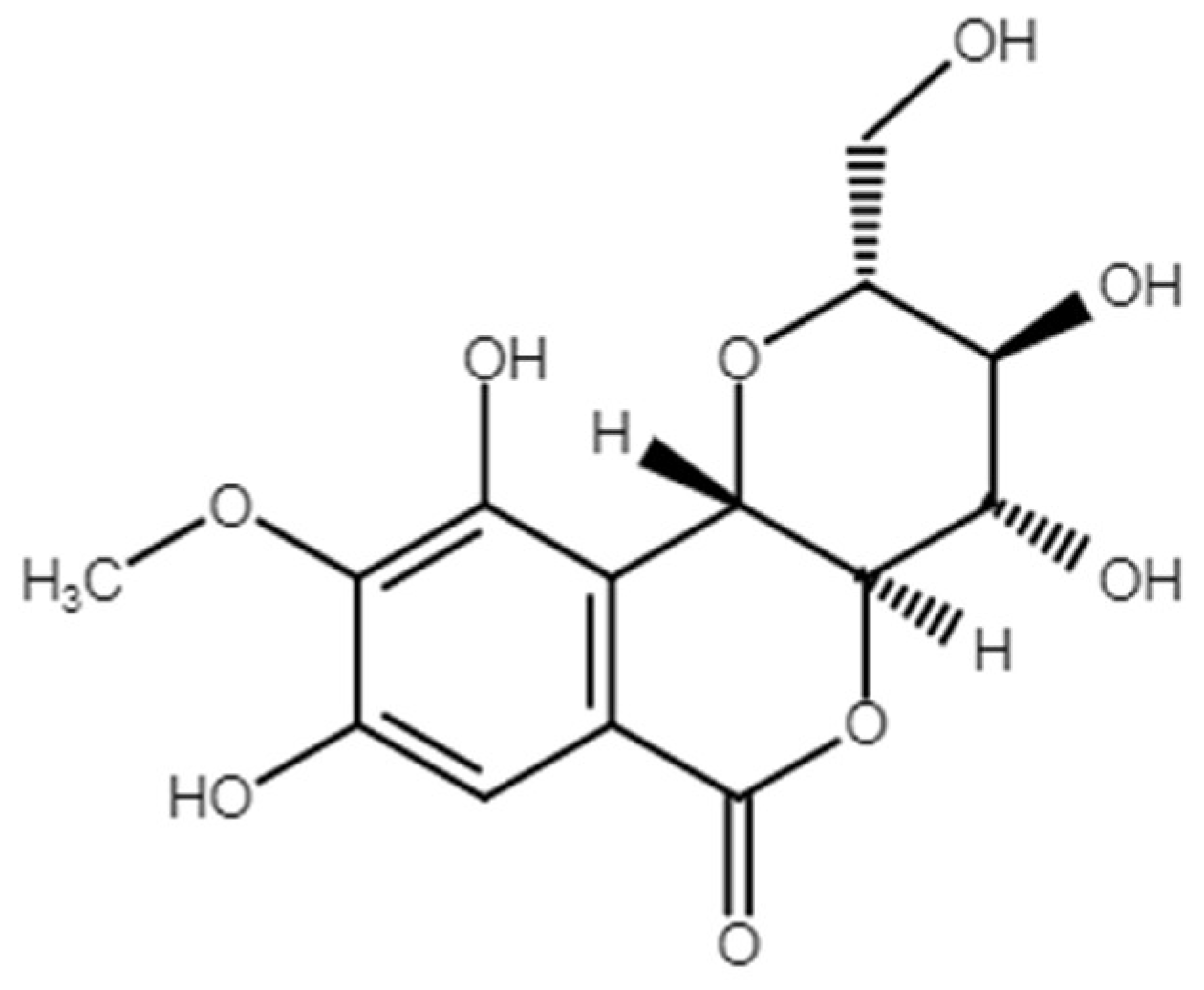
4.2. Bergenin
4.3. Mouse Model of STZ-Induced Diabetes
4.4. Experimental Design
4.5. Assessment of Sensory Neuropathy by Von Frey Filaments
4.6. The Rota-Rod Test for Assessment of Motor Impairment
4.7. In Vitro Assays: Cytotoxicity and Nitrite Levels in Macrophage Cultures
4.8. Estimation of Lipid Peroxidation and Nitrite in Nervous Tissues
4.9. Real-Time PCR
4.10. Quantification of Cytokines Levels by ELISA
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation end product |
| BER | Bergenin |
| CTRL | Control |
| DEXA | Dexamethasone |
| DMEM | Dulbecco’s modified Eagle medium |
| DMSO | Dimethyl sulfoxide |
| DN | Diabetic neuropathy |
| DRG | Dorsal root ganglia |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| GABA | Gabapentin |
| Gpx | Glutathione peroxidase |
| GV | Gentian violet |
| IFN-γ | Interferon-γ |
| IL-1β | Interleukin-1β |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MDA | Malondialdehyde |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PBS | Phosphate buffered saline |
| ROS | Reactive oxygen species |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| STZ | Streptozotocin |
| TBARS | Thiobarbituric acid-reactive substances |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-α |
| VEH | Vehicle |
References
- Chaturvedi, N. The burden of diabetes and its complications: Trends and implications for intervention. Diabetes Res. Clin. Pract. 2007, 76, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, J.W.; Pop-Busui, R. Diabetic neuropathy: Mechanisms, emerging treatments, and subtypes. Curr. Neurol. Neurosci. Rep. 2014, 14, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfaye, S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J. Diabetes Investig. 2011, 2, 33–42. [Google Scholar] [CrossRef]
- Khdour, M.R. Treatment of diabetic peripheral neuropathy: A review. J. Pharm. Pharmacol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Waldfogel, J.M.; Nesbit, S.A.; Dy, S.M.; Sharma, R.; Zhang, A.; Wilson, L.M.; Bennett, W.L.; Yeh, H.C.; Chelladurai, Y.; Feldman, D.; et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: A systematic review. Neurology 2017, 88, 1958–1967. [Google Scholar] [CrossRef]
- Evangelista, A.F.; Vannier-Santos, M.A.; de Assis Silva, G.S.; Silva, D.N.; Juiz, P.J.L.; Nonaka, C.K.V.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J. Neuroinflammation 2018, 15, 189. [Google Scholar] [CrossRef]
- Galuppo, M.; Giacoppo, S.; Bramanti, P.; Mazzon, E. Use of natural compounds in the management of diabetic peripheral neuropathy. Molecules 2014, 19, 2877–2895. [Google Scholar] [CrossRef] [Green Version]
- Abe, K.; Sakai, K.; Uchida, M. Effects of bergenin on experimental ulcers—Prevention of stress induced ulcers in rats. Gen. Pharmacol. Vasc. Syst. 1980, 11, 361–368. [Google Scholar] [CrossRef]
- Lim, H.-K.; Kim, H.-S.; Choi, H.-S.; Oh, S.; Choi, J. Hepatoprotective effects of bergenin, a major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated rats. J. Ethnopharmacol. 2000, 72, 469–474. [Google Scholar] [CrossRef]
- Xiang, S.; Chen, K.; Xu, L.; Wang, T.; Guo, C. Bergenin Exerts Hepatoprotective Effects by Inhibiting the Release of Inflammatory Factors, Apoptosis and Autophagy via the PPAR-gamma Pathway. Drug Des. Dev. Ther. 2020, 14, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Xu, M.; Luo, K.; Huang, W.; Yu, H.; Zhou, T. Anticancer activity of bergenin against cervical cancer cells involves apoptosis, cell cycle arrest, inhibition of cell migration and the STAT3 signalling pathway. Exp. Ther. Med. 2019, 17, 3525–3529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barai, P.; Raval, N.; Acharya, S.; Borisa, A.; Bhatt, H.; Acharya, N. Neuroprotective effects of bergenin in Alzheimer’s disease: Investigation through molecular docking, in vitro and in vivo studies. Behav. Brain Res. 2019, 356, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, D.; Zhang, B.; Lu, H. Bergenin Ameliorates MPTP-Induced Parkinson’s Disease by Activating PI3K/Akt Signaling Pathway. J. Alzheimer’s Dis. 2019, 72, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Patel, D.K.; Prasad, S.K.; Laloo, D.; Krishnamurthy, S.; Hemalatha, S. Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler. Fitoterapia 2012, 83, 395–401. [Google Scholar] [CrossRef]
- de Oliveira, C.M.; Nonato, F.R.; de Lima, F.O.; Couto, R.D.; David, J.P.; David, J.M.; Soares, M.B.; Villarreal, C.F. Antinociceptive properties of bergenin. J. Nat. Prod. 2011, 74, 2062–2068. [Google Scholar] [CrossRef]
- Gao, X.J.; Guo, M.Y.; Zhang, Z.C.; Wang, T.C.; Cao, Y.G.; Zhang, N.S. Bergenin Plays an Anti-Inflammatory Role via the Modulation of MAPK and NF-kappaB Signaling Pathways in a Mouse Model of LPS-Induced Mastitis. Inflammation 2015, 38, 1142–1150. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, C.; Kaushik, S.R.; Kulshreshtha, A.; Chaturvedi, S.; Nanda, R.K.; Bhaskar, A.; Chattopadhyay, D.; Das, G.; Dwivedi, V.P. The phytochemical bergenin as an adjunct immunotherapy for tuberculosis in mice. J. Biol. Chem. 2019, 294, 8555–8563. [Google Scholar] [CrossRef]
- Lopes de Oliveira, G.A.; Alarcon de la Lastra, C.; Rosillo, M.A.; Castejon Martinez, M.L.; Sanchez-Hidalgo, M.; Rolim Medeiros, J.V.; Villegas, I. Preventive effect of bergenin against the development of TNBS-induced acute colitis in rats is associated with inflammatory mediators inhibition and NLRP3/ASC inflammasome signaling pathways. Chem. Biol. Interact. 2019, 297, 25–33. [Google Scholar] [CrossRef]
- Ren, X.; Ma, S.; Wang, J.; Tian, S.; Fu, X.; Liu, X.; Li, Z.; Zhao, B.; Wang, X. Comparative effects of dexamethasone and bergenin on chronic bronchitis and their anti-inflammatory mechanisms based on NMR metabolomics. Mol. Biosyst. 2016, 12, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, B.; Zhang, C.; Qiu, M.; Ma, S.; Jin, X.; Shao, Y.; Wang, M.; Wang, X. Mechanisms of bergenin treatment on chronic bronchitis analyzed by liquid chromatography-tandem mass spectrometry based on metabolomics. Biomed. Pharm. 2019, 109, 2270–2277. [Google Scholar] [CrossRef]
- Alves, C.Q.; David, J.M.; David, J.P.; Villareal, C.F.; Soares, M.B.P.; Queiroz, L.P.D.; Aguiar, R.M. Flavonoids and other bioactive phenolics isolated from Cenostigma macrophyllum (Leguminosae). Química Nova 2012, 35, 1137–1140. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, G.A.L.; da Silva Oliveira, G.L.; Nicolau, L.A.D.; Mafud, A.C.; Batista, L.F.; Mascarenhas, Y.P.; de Sousa, L.K.M.; David, J.M.; Pinto, L.S.; Alves, C.Q.; et al. Bergenin from Peltophorum dubium: Isolation, Characterization, and Antioxidant Activities in Non-Biological Systems and Erythrocytes. Med. Chem. 2017, 13, 592–603. [Google Scholar] [CrossRef]
- Qi, Q.; Dong, Z.; Sun, Y.; Li, S.; Zhao, Z. Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules 2018, 23, 2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, S.; Liu, R.; Lv, C.; Miao, Y.; Yue, M.; Tao, Y.; Wei, Z.; Xia, Y.; Dai, Y. Bergenin impedes the generation of extracellular matrix in glomerular mesangial cells and ameliorates diabetic nephropathy in mice by inhibiting oxidative stress via the mTOR/beta-TrcP/Nrf2 pathway. Free Radic. Biol. Med. 2019, 145, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lee, Y.; Yun, K.; Oh, S. Bergenin decreases the morphine-induced physical dependence via antioxidative activity in mice. Arch. Pharmacal Res. 2015, 38, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012, 11, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Rakieten, N.; Rakieten, M.L.; Nadkarni, M.V. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep. 1963, 29, 91–98. [Google Scholar]
- Yang, J.; Kan, M.; Wu, G.Y. Bergenin ameliorates diabetic nephropathy in rats via suppressing renal inflammation and TGF-beta1-Smads pathway. Immunopharmacol. Immunotoxicol. 2016, 38, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Li, Y.F.; Lv, Q.; Li, X.M.; Dai, Y.; Wei, Z.F. Bergenin, Acting as an Agonist of PPARgamma, Ameliorates Experimental Colitis in Mice through Improving Expression of SIRT1, and Therefore Inhibiting NF-kappaB-Mediated Macrophage Activation. Front. Pharmacol. 2017, 8, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Kim, H.J.; Lee, Y.S.; Kang, G.M.; Lim, H.S.; Lee, S.H.; Song, D.K.; Kwon, O.; Hwang, I.; Son, M.; et al. Hypothalamic Macrophage Inducible Nitric Oxide Synthase Mediates Obesity-Associated Hypothalamic Inflammation. Cell Rep. 2018, 25, 934–946.e5. [Google Scholar] [CrossRef] [Green Version]
- Schmidtko, A. Nitric oxide-mediated pain processing in the spinal cord. Handb. Exp. Pharmacol. 2015, 227, 103–117. [Google Scholar] [PubMed]
- Staunton, C.A.; Barrett-Jolley, R.; Djouhri, L.; Thippeswamy, T. Inducible nitric oxide synthase inhibition by 1400W limits pain hypersensitivity in a neuropathic pain rat model. Exp. Physiol. 2018, 103, 535–544. [Google Scholar] [CrossRef]
- Ahlawat, A.; Sharma, S. A new promising simultaneous approach for attenuating type II diabetes mellitus induced neuropathic pain in rats: iNOS inhibition and neuroregeneration. Eur. J. Pharmacol. 2018, 818, 419–428. [Google Scholar] [CrossRef]
- Vincent, A.M.; Russell, J.W.; Low, P.; Feldman, E.L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr. Rev. 2004, 25, 612–628. [Google Scholar] [CrossRef]
- Cellek, S.; Qu, W.; Schmidt, A.M.; Moncada, S. Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: A new insight into selective nitrergic neuropathy in diabetes. Diabetologia 2004, 47, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Strom, J.; Xu, B.; Tian, X.; Chen, Q.M. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016, 30, 66–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Hu, X.; Liang, F.; Liu, J.; Zhou, H.; Liu, J.; Wang, H.; Tang, H. Therapeutic effects of moxibustion simultaneously targeting Nrf2 and NF-kappaB in diabetic peripheral neuropathy. Appl. Biochem. Biotechnol. 2019, 189, 1167–1182. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [Green Version]
- Erbas, O.; Taskiran, D.; Oltulu, F.; Yavasoglu, A.; Bora, S.; Bilge, O.; Cinar, B.P.; Peker, G. Oxytocin provides protection against diabetic polyneuropathy in rats. Neurol. Res. 2017, 39, 45–53. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Ye, R.; He, Y.; Li, Y.; Qiu, X. Protective effect of Jiaweibugan decoction against diabetic peripheral neuropathy. Neural Regen. Res. 2013, 8, 1113–1121. [Google Scholar]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [Green Version]
- Hung, A.L.; Lim, M.; Doshi, T.L. Targeting cytokines for treatment of neuropathic pain. Scand. J. Pain 2017, 17, 287–293. [Google Scholar] [CrossRef]
- Safieh-Garabedian, B.; Nomikos, M.; Saade, N. Targeting inflammatory components in neuropathic pain: The analgesic effect of thymulin related peptide. Neurosci. Lett. 2019, 702, 61–65. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Yu, Z.; Wang, L.; Yuan, T.; Wang, X.; Zhang, X.; Wang, J.; Lv, Y.; Du, G. The natural product bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-kappaB activition. J. Ethnopharmacol. 2017, 200, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.B.; Ramana, K.V.; Srivastava, S.; Bhatnagar, A.; Srivastava, S.K. Aldose reductase regulates high glucose-induced ectodomain shedding of tumor necrosis factor (TNF)-alpha via protein kinase C-delta and TNF-alpha converting enzyme in vascular smooth muscle cells. Endocrinology 2009, 150, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Macedo, F.H.P.; Aires, R.D.; Fonseca, E.G.; Ferreira, R.C.M.; Machado, D.P.D.; Chen, L.; Zhang, F.X.; Souza, I.A.; Lemos, V.S.; Romero, T.R.L.; et al. TNF-alpha mediated upregulation of NaV1.7 currents in rat dorsal root ganglion neurons is independent of CRMP2 SUMOylation. Mol. Brain 2019, 12, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L.; et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.C.; Stemkowski, P.L.; Smith, P.A. Long-term actions of interleukin-1beta on K(+), Na(+) and Ca(2+) channel currents in small, IB4-positive dorsal root ganglion neurons; possible relevance to the etiology of neuropathic pain. J. Neuroimmunol. 2019, 332, 198–211. [Google Scholar] [CrossRef]
- Sandireddy, R.; Yerra, V.G.; Areti, A.; Komirishetty, P.; Kumar, A. Neuroinflammation and oxidative stress in diabetic neuropathy: Futuristic strategies based on these targets. Int. J. Endocrinol. 2014, 2014, 674987. [Google Scholar] [CrossRef] [Green Version]
- Benveniste, E.N.; Tang, L.P.; Law, R.M. Differential regulation of astrocyte TNF-alpha expression by the cytokines TGF-beta, IL-6 and IL-10. Int. J. Dev. Neurosci. 1995, 13, 341–349. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-beta Signaling. Biomolecules 2020, 10, E487. [Google Scholar] [CrossRef] [Green Version]
- Blobe, G.C.; Schiemann, W.P.; Lodish, H.F. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 2000, 342, 1350–1358. [Google Scholar] [CrossRef]
- Chen, N.F.; Huang, S.Y.; Lu, C.H.; Chen, C.L.; Feng, C.W.; Chen, C.H.; Hung, H.C.; Lin, Y.Y.; Sung, P.J.; Sung, C.S.; et al. Flexibilide obtained from cultured soft coral has anti-neuroinflammatory and analgesic effects through the upregulation of spinal transforming growth factor-beta1 in neuropathic rats. Mar. Drugs 2014, 12, 3792–3817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echeverry, S.; Shi, X.Q.; Haw, A.; Liu, H.; Zhang, Z.W.; Zhang, J. Transforming growth factor-beta1 impairs neuropathic pain through pleiotropic effects. Mol. Pain 2009, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Guimarães, E.T.; Cruz Gda, S.; Almeida, T.F.; Souza, B.S.; Kaneto, C.M.; Vasconcelos, J.F.; Santos, W.L.; Santos, R.R.; Villarreal, C.F.; Soares, M.B. Transplantation of stem cells obtained from murine dental pulp improves pancreatic damage, renal function, and painful diabetic neuropathy in diabetic type 1 mouse model. Cell Transplant. 2013, 22, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Reda, H.M.; Zaitone, S.A.; Moustafa, Y.M. Effect of levetiracetam versus gabapentin on peripheral neuropathy and sciatic degeneration in streptozotocin-diabetic mice: Influence on spinal microglia and astrocytes. Eur. J. Pharmacol. 2016, 771, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.J. The Up-and-Down Method for Small Samples. J. Am. Stat. Assoc. 1965, 60, 967–978. [Google Scholar] [CrossRef]
- Leite dos Santos, G.G.; Casais e Silva, L.L.; Pereira Soares, M.B.; Villarreal, C.F. Antinociceptive properties of Micrurus lemniscatus venom. Toxicon 2012, 60, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Espirito-Santo, R.F.; Meira, C.S.; Costa, R.D.S.; Souza Filho, O.P.; Evangelista, A.F.; Trossini, G.H.G.; Ferreira, G.M.; Velozo, E.D.S.; Villarreal, C.F.; Pereira Soares, M.B. The anti-inflammatory and immunomodulatory potential of braylin: Pharmacological properties and mechanisms by in silico, in vitro and in vivo approaches. PLoS ONE 2017, 12, e0179174. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Tiwari, V.; Kuhad, A.; Chopra, K. Tocotrienol ameliorates behavioral and biochemical alterations in the rat model of alcoholic neuropathy. Pain 2009, 145, 129–135. [Google Scholar] [CrossRef]
- Gama, K.B.; Santos, D.S.; Evangelista, A.F.; Silva, D.N.; de Alcantara, A.C.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation. Stem Cells Int. 2018, 2018, 8179013. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarreal, C.F.; Santos, D.S.; Lauria, P.S.S.; Gama, K.B.; Espírito-Santo, R.F.; Juiz, P.J.L.; Alves, C.Q.; David, J.M.; Soares, M.B.P. Bergenin Reduces Experimental Painful Diabetic Neuropathy by Restoring Redox and Immune Homeostasis in the Nervous System. Int. J. Mol. Sci. 2020, 21, 4850. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144850
Villarreal CF, Santos DS, Lauria PSS, Gama KB, Espírito-Santo RF, Juiz PJL, Alves CQ, David JM, Soares MBP. Bergenin Reduces Experimental Painful Diabetic Neuropathy by Restoring Redox and Immune Homeostasis in the Nervous System. International Journal of Molecular Sciences. 2020; 21(14):4850. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144850
Chicago/Turabian StyleVillarreal, Cristiane F., Dourivaldo S. Santos, Pedro S. S. Lauria, Kelly B. Gama, Renan F. Espírito-Santo, Paulo J. L. Juiz, Clayton Q. Alves, Jorge M. David, and Milena B. P. Soares. 2020. "Bergenin Reduces Experimental Painful Diabetic Neuropathy by Restoring Redox and Immune Homeostasis in the Nervous System" International Journal of Molecular Sciences 21, no. 14: 4850. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144850





