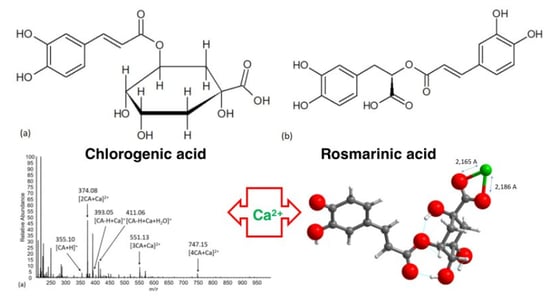
Interactions of Calcium with Chlorogenic and Rosmarinic Acids: An Experimental and Theoretical Approach
Abstract
:1. Introduction
2. Results
2.1. Spectroscopic Studies of Calcium Interaction with RA and CA
2.1.1. UV-Visible Spectroscopy
2.1.2. Electrospray Mass Spectrometry
2.1.3. NMR Spectroscopy
2.2. Density Functional Theory Calculations
2.3. Effect of Calcium on Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Spectroscopic Studies
4.3. Molecular Modeling
4.4. Antioxidant Activity Assays
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CA | Chlorogenic acid, (1S,3R,4R,5R)-3-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-1,4,5- trihydroxycyclohexanecarboxylic acid |
| DFT DPPH TRIS | Density functional theory 2,2-diphenyl-1-picrylhydrazyl tris(hydroxymethyl)aminomethane |
| RA | Rosmarinic acid, (2R)-3-(3,4-dihydroxyphenyl)-2-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl] oxy}propanoic acid |
References
- World Health Organization (WHO). Global Report on Traditional and Complimentary Medicine; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Prochazkova, K.; Boušová, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Salami, M.; Rahimmalek, M.; Ehtemam, M.H. Inhibitory effect of different fennel (Foeniculum vulgare) samples and their phenolic compounds on formation of advanced glycation products and comparison of antimicrobial and antioxidant activities. Food Chem. 2016, 213, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-inflammatory and Antioxidant properties of Red Grape Polyphenols: In Vitro and in Vivo Studies. Antioxidants 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalova, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef]
- Říha, M.; Karlíčková, J.; Filipský, T.; Macakova, K.; Rocha, L.; Bovicelli, P.; Silvestri, I.P.; Saso, L.; Jahodář, L.; Hrdina, R.; et al. In vitro evaluation of copper-chelating properties of flavonoids. RSC Adv. 2014, 4, 32628–32638. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, M.-Q. Studies on Transition Metal-Quercetin Complexes Using Electrospray Ionization Tandem Mass Spectrometry. Molecules 2015, 20, 8583–8594. [Google Scholar] [CrossRef] [PubMed]
- Marković, J.M.D.; Markovic, Z.; Brdaric, T.; Pavelkić, V.M.; Jadranin, M.B. Iron complexes of dietary flavonoids: Combined spectroscopic and mechanistic study of their free radical scavenging activity. Food Chem. 2011, 129, 1567–1577. [Google Scholar] [CrossRef]
- Perez, C.A.; Wei, Y.; Guo, M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorg. Biochem. 2009, 103, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trofimova, N.N.; Stolpovskaya, E.V.; Babkin, V.A.; Fedorov, S.V.; Kalabin, G.A.; Goryainov, S.V.; Zolotarev, E.E.; Safronovc, A.Y.; Kashevskii, A.V.; Zhitov, R.G. The structure and electrochemical properties of metal complexes with dihydroquercetin. Russ. J. Bioorg. Chem. 2015, 41, 745–752. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Liu, H.-Y.; Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Genistein Binding to Copper (II)—Solvent Dependence and Effects on Radical Scavenging. Molecules 2017, 22, 1757. [Google Scholar] [CrossRef] [Green Version]
- Panhwar, Q.K.; Memon, S.; Bhanger, M. Synthesis, characterization, spectroscopic and antioxidation studies of Cu (II)—Morin complex. J. Mol. Struct. 2010, 967, 47–53. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 757–771. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence, and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Pollini, L.; Rocchi, R.; Cossignani, L.; Mañes, J.; Compagnone, D.; Blasi, F. Phenol Profiling and Nutraceutical Potential of Lycium spp. Leaf Extracts Obtained with Ultrasound and Microwave Assisted Techniques. Antioxidants 2019, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Kalinowska, M.; Bajko, E.; Matejczyk, M.; Kaczynski, P.; Łozowicka, B.; Lewandowski, W. The Study of Anti-/Pro-Oxidant, Lipophilic, Microbial and Spectroscopic Properties of New Alkali Metal Salts of 5-O-Caffeoylquinic Acid. Int. J. Mol. Sci. 2018, 19, 463. [Google Scholar] [CrossRef] [Green Version]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef] [Green Version]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant Properties of Ferulic Acid, and Its Related Compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.B.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. 2020, 34, 685–720. [Google Scholar] [CrossRef]
- Milić, S.Z.; Potkonjak, N.; Gorjanović, S.Ž.; Jovanovic, S.V.; Pastor, F.T.; Sužnjević, D.Ž. A Polarographic Study of Chlorogenic Acid and Its Interaction with Some Heavy Metal Ions. Electroanalytical 2011, 23, 2935–2940. [Google Scholar] [CrossRef]
- Beneduci, A.; Furia, E.; Russo, N.; Marino, T. Complexation behaviour of caffeic, ferulic and p-coumaric acids towards aluminium cations: A combined experimental and theoretical approach. New J. Chem. 2017, 41, 5182–5190. [Google Scholar] [CrossRef]
- Chan, S.; Kantham, S.; Rao, V.M.; Palanivelu, M.K.; Pham, H.L.; Shaw, P.N.; McGeary, R.P.; Ross, B.P. Metal chelation, radical scavenging, and inhibition of Aβ42 fibrillation by food constituents in relation to Alzheimer’s disease. Food Chem. 2016, 199, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Kutus, B.; Ozsvár, D.; Varga, N.; Pálinkó, I.; Sipos, P. ML and ML 2 complex formation between Ca (II) and d -glucose derivatives in aqueous solutions. Dalton Trans. 2017, 46, 1065–1074. [Google Scholar] [CrossRef]
- Gao, L.-G.; Wang, H.; Song, X.-L.; Cao, W. Research on the chelation between luteolin and Cr (III)-ion through infrared spectroscopy, UV–vis spectrum and theoretical calculations. J. Mol. Struct. 2013, 1034, 386–391. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E.; Kalinowska, M. Hydroxyflavone metal complexes—Molecular structure, antioxidant activity and biological effects. Chem. Interact. 2017, 273, 245–256. [Google Scholar] [CrossRef]
- Cornard, J.-P.; Lapouge, C.; Dangleterre, L.; Allet-Bodelot, C. Complexation of Lead (II) by Chlorogenic Acid: Experimental and Theoretical Study. J. Phys. Chem. A 2008, 112, 12475–12484. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, R. Study of Complexes of Tannic Acid with Fe (III) and Fe (II). J. Anal. Methods Chem. 2019, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Al-Danaf, N.; Melhem, R.A.; Assaf, K.I.; Nau, W.M.; Patra, D. Photophysical properties of neutral and dissociated forms of rosmarinic acid. J. Lumin 2016, 175, 50–56. [Google Scholar] [CrossRef]
- Paulpandi, R.Q.; Ramasamy, S.; Paulraj, M.S.; Baños, F.G.D.; Víllora, G.; Cerón-Carrasco, J.P.; Pérez-Sánchez, H.; Enoch, I.V.M.V. Enhanced Zn2+ion-sensing behavior of a benzothiazole derivative on encapsulation by β-cyclodextrin. RSC Adv. 2016, 6, 15670–15677. [Google Scholar] [CrossRef] [Green Version]
- Kelley, C.J.; Harruff, R.C.; Carmack, M. Polyphenolic acids of Lithospermum ruderale. II. Carbon-13 nuclear magnetic resonance of lithospermic and rosmarinic acids. J. Org. Chem. 1976, 41, 449–455. [Google Scholar] [CrossRef]
- Zapata, J.M.; López-Arnaldos, T.; López-Serrano, M.; Barceló, A.R.; Calderón, A. Spectrophotometric determination of rosmarinic acid in plant cell cultures by complexation with Fe2+ ions. Anal. Bioanal. Chem. 1995, 351, 311–314. [Google Scholar] [CrossRef]
- Castillo-Blum, S.E.; Barba-Behrens, N. Coordination chemistry of some biologically active ligands. Co-Ord. Chem. Rev. 2000, 196, 3–30. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta Bioenerg. 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Cheynier, V.; Tomás-Barberán, F.A.; Yoshida, K. Polyphenols: From Plants to a Variety of Food and Nonfood Uses. J. Agric. Food Chem. 2015, 63, 7589–7594. [Google Scholar] [CrossRef]
- Quero, J.; Marmol, I.; Cerrada, E.; Rodriguez-Yoldi, M.J. Insight into the potential application of polyphenol-rich dietary intervention in degenerative disease management. Food Funct. 2020, 11, 2805–2825. [Google Scholar] [CrossRef]
- Olszewka, M.A.; Gedas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.; Ochocki, J.; Erxleben, A. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Dandawate, P.; Padhye, S.; Schobert, R.; Biersack, B. Discovery of natural products with metal-binding properties as promising antibacterial agents. Expert Opin. Drug Discov. 2019, 14, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Mila, I.; Expert, D.; Marmolle, F.; Albrecht, A.-M.; Hurrell, R.; Huneau, J.-F.; Tomé, D. Polyphenols, metal ion complexation and biological consequences. In Basic Life Sciences; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1999; Volume 66, pp. 545–554. [Google Scholar]
- Murakami, K.; Haneda, M.; Qiao, S.; Naruse, M.; Yoshino, M. Prooxidant action of rosmarinic acid: Transition metal-dependent generation of reactive oxygen species. Toxicol. Vitr. 2007, 21, 613–617. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Westervelt, H.H.; Frederick, W.; Malcolm, E.W.; Easty, D.B. The determination and temperature-dependence of the stability constant of the calcium—catechol-4-sulfonate complex in alkaline aqueous media. Anal. Chim. Acta 1982, 138, 237–243. [Google Scholar] [CrossRef]
- Świsłocka, R.; Regulska, E.; Karpinska, J.; Świderski, G.; Lewandowski, W. Molecular Structure and Antioxidant Properties of Alkali Metal Salts of Rosmarinic Acid. Experimental and DFT Studies. Molecules 2019, 24, 2645. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- ArrayExpress—A Database of Functional Genomics Experiments. Available online: http://www.ebi.ac.uk/arrayexpress/ (accessed on 12 November 2012).
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickard, C.J.; Mauri, F. All-electron magnetic response with pseudopotentials: NMR chemical shifts. Phys. Rev. B 2001, 63, 245101. [Google Scholar] [CrossRef] [Green Version]
- Yates, J.R.; Pickard, C.J.; Mauri, F. Calculation of NMR chemical shifts for extended systems using ultrasoft pseudopotentials. Phys. Rev. B 2007, 76, 024401. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations using a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]







| Configuration | Deprotonation Site(s) | Complexation Site(s) | Energy (eV) |
|---|---|---|---|
| Chlorogenic acid | |||
| CAI | C8–C20 | C8 | −266.07 |
| CAII | C19–C20 | −265.12 | |
| Rosmarinic acid | |||
| RAI | C14–C3 | C14 | −270.97 |
| RAII | C3–C4 | −268.86 | |
| RAIII | C14–C20 | C14 | −269.29 |
| RAIV | C19–C20 | −268.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palierse, E.; Przybylski, C.; Brouri, D.; Jolivalt, C.; Coradin, T. Interactions of Calcium with Chlorogenic and Rosmarinic Acids: An Experimental and Theoretical Approach. Int. J. Mol. Sci. 2020, 21, 4948. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144948
Palierse E, Przybylski C, Brouri D, Jolivalt C, Coradin T. Interactions of Calcium with Chlorogenic and Rosmarinic Acids: An Experimental and Theoretical Approach. International Journal of Molecular Sciences. 2020; 21(14):4948. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144948
Chicago/Turabian StylePalierse, Estelle, Cédric Przybylski, Dalil Brouri, Claude Jolivalt, and Thibaud Coradin. 2020. "Interactions of Calcium with Chlorogenic and Rosmarinic Acids: An Experimental and Theoretical Approach" International Journal of Molecular Sciences 21, no. 14: 4948. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144948