Building on Surface-Active Ionic Liquids for the Rescuing of the Antimalarial Drug Chloroquine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis and Thermal Stability
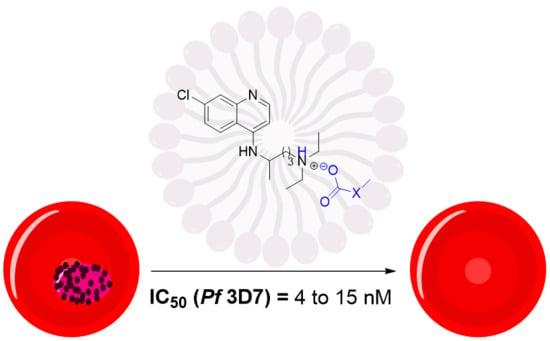
2.2. Antimalarial Activity In Vitro
2.3. Surface Tension Studies
3. Concluding Remarks
4. Materials and Methods
4.1. Chemical Synthesis
4.1.1. Conversion of Chloroquine Phosphate into 1a
4.1.2. Synthesis of Chloroquine Analogue 1b
4.1.3. Synthesis of Ionic Liquids 3
4.1.4. Synthesis of Amides 4
4.2. Simultaneous Thermogravimetric Analysis
4.3. Surface Tension Measurements
4.4. In Vitro Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| CQ | Chloroquine |
| CTAB | Cetyltrimethylammonium bromide |
| DCM | Dichloromethane |
| DIEA | N-ethyl-N,N-diisopropylamine |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| DMSO-d6 | Hexadeuterated dimethylsulfoxide |
| eq | Molar equivalent |
| ESI-IT MS | Electrospray ionization-ion trap mass spectrometry |
| IC50 | Half-maximal inhibitory concentration |
| IL | Ionic liquid |
| MeOH | Methanol |
| NMR | Nuclear magnetic resonance |
| Pf | Plasmodium falciparum |
| PQ | Primaquine |
| RT | Room temperature |
| RTIL | Room temperature ionic liquid |
| SAIL | Surface-active ionic liquid |
| SD | Standard deviation |
| SM | Supplementary materials |
| STA | Simultaneous thermogravimetric analysis |
| TBTU | O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate |
References
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic liquids in pharmaceutical applications. Ann. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Cláudio, A.F.M.; Valega, M.; Domingues, F.M.J.; Silvestre, A.J.D.; Rogers, R.D.; Coutinho, J.A.P.; Freire, M.G. Switchable (pH-driven) aqueous biphasic systems formed by ionic liquids as integrated production–separation platforms. Green Chem. 2017, 19, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Almeida, M.R.; Silva, F.A.; Domingues, P.; Ventura, S.P.M.; Coutinho, J.A.P.; Freire, M.G. Novel biocompatible and self-buffering ionic liquids for biopharmaceutical applications. Chem. Eur. J. 2015, 21, 4781–4788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamshina, J.L.; Rogers, R.D. Overcoming the problems of solid state drug formulations with ionic liquids. Ther. Deliv. 2014, 5, 489–491. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, R.; Teixeira, V.; Rodrigues, D.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Antibacterial activity of ionic liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef] [Green Version]
- Pérez, B.; Teixeira, C.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.B.; Gomes, P. Cinnamic acid/chloroquinoline conjugates as potent agents against chloroquine-resistant Plasmodium falciparum. Chem. Med. Chem. 2012, 7, 1537–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.; Pérez, B.; Albuquerque, I.; Machado, M.; Nogueira, F.; Prudêncio, M.; Teixeira, C.; Gomes, P. N-cinnamoylation of antimalarial classics: Quinacrine analogues with decreased toxicity and dual-stage activity. Chem. Med. Chem. 2014, 9, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Machado, M.; Lobo, L.; Nogueira, F.; Prudêncio, M.; Teixeira, C.; Gomes, P. N-Cinnamoylation of antimalarial classics: Effects of using acyl groups other than cinnamoyl toward dual-stage antimalarials. Chem. Med. Chem. 2015, 10, 1344–1349. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Fernandes, I.; Teixeira, C.; Mateus, N.; Sottomayor, M.J.; Gomes, P. A quinacrine analogue selective against gastric cancer cells: Insight from biochemical and biophysical studies. Chem. Med. Chem. 2016, 11, 2703–2712. [Google Scholar] [CrossRef]
- Ferraz, R.; Noronha, J.; Murtinheira, F.; Nogueira, F.; Machado, M.; Prudêncio, M.; Parapini, S.; D’Alessandro, S.; Teixeira, C.; Gomes, A.; et al. Primaquine-based ionic liquids as a novel class of antimalarial hits. RSC Adv. 2016, 6, 56134–56138. [Google Scholar] [CrossRef]
- Teixeira, C.; Vale, N.; Pérez, B.; Gomes, A.; Gomes, J.R.B.; Gomes, P. “Recycling” classical drugs for malaria. Chem. Rev. 2014, 114, 11164–11220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraz, R.; Pinheiro, M.; Teixeira, C.; Gomes, A.; Prudêncio, C.; Reis, S.; Gomes, A. Effects of novel triple-stage antimalarial ionic liquids on lipid membrane models. Bioorg. Med. Chem. Lett. 2017, 27, 4190–4193. [Google Scholar] [CrossRef] [PubMed]
- Tay, E.; Nguyen, T.-H.; Ford, L.; Williams, H.D.; Benameur, H.; Scammells, P.J.; Porter, C.J.H. Ionic liquid forms of the antimalarial lumefantrine in combination with LFCS Type IIIB lipid-based formulations preferentially increase lipid solubility, in vitro solubilization behavior and in vivo exposure. Pharmaceutics 2020, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Stoimenovski, J.; Izgorodina, E.I.; MacFarlane, D.R. Ionicity and proton transfer in protic ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 10341–10347. [Google Scholar] [CrossRef]
- Fameau, A.-L.; Arnould, A.; Lehmann, M.; von Klitzing, R. Photoresponsive self-assemblies based on fatty acids. Chem. Commun. 2015, 51, 2907–2910. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Kaur, M.; Drechsler, M.; Kang, T.S. Unprecedented self-assembled architectures of surface-active ionic liquids in aqueous medium. Chem. Commun. 2018, 54, 2432–2435. [Google Scholar] [CrossRef]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline versus ionic liquid salt forms of active pharmaceutical ingredients: A position paper. Pharm. Res. 2009, 27, 521–526. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Santos, M.M.; Raposo, L.R.; Carrera, G.V.S.M.; Costa, A.; Dionísio, M.; Baptista, P.V.; Fernandes, A.R.; Branco, L.C. Ionic liquids and salts from ibuprofen as promising innovative formulations of an old drug. Chem. Med. Chem. 2019, 14, 907–911. [Google Scholar] [CrossRef]
- Nogueira, F.; Diez, A.; Radfar, A.; Pérez-Benavente, S.; do Rosário, V.E.; Puyet, A.; Bautista, J.M. Early transcriptional response to chloroquine of the Plasmodium falciparum antioxidant defence in sensitive and resistant clones. Acta Trop. 2010, 114, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Murtinheira, F.; Lobo, E.N.F. Whole-cell SYBR Green I assay for antimalarial activity assessment. Ann. Clin. Med. Microbiol. 2016, 2, 1010. [Google Scholar]


| Compound | Synthesis Yield/% | Temperature of Degradation Events Observed/°C | Half-Maximal Inhibitory Concentration (IC50) ± SD/nM | |
|---|---|---|---|---|
| Pf 3D7 | Pf Dd2 | |||
| 3a | 99 | 93.2; 236.9 | 12 ± 5 | 384 ± 142 |
| 3b | 99 | 120.7; 220.5 | 13 ± 2 | 402 ± 190 |
| 3c | 88 | 156.9; 228.5 | 4 ± 1 | 110 ± 36 |
| 3d | 98 | 227.7 | 12 ± 5 | 235 ± 79 |
| 3e | 99 | 197.1 | 15 ± 4 | 365 ± 126 |
| 4a | 75 | 291.3 | 627 ± 142 | 588 ± 44 |
| 4b | 66 | 314.1 | 51 ± 9 | 109 ± 7 |
| 4c | 74 | 310.8 | 70 ± 7 | 160 ± 14 |
| 4d | 70 | 335.2 | n.d. 2 | n.d. 2 |
| 4e | 48 | 336.5 | n.d. 2 | n.d. 2 |
| 2a | − | 75.5 | n.d. | n.d. |
| 2b | − | 131.0 | n.d. | n.d. |
| 2c | − | 172.3 | >10,000 | >10,000 |
| 2d | − | 218.5 | n.d. | n.d. |
| 2e | − | 211.6 | n.d. | n.d. |
| CQ 1 | − | 301.7 | 45 ± 15 | 660 ± 11 |
| CQ 1 + 2c | − | − | 59 ± 16 | 415 ± 44 |
| System | cmc/mmol·kg−1 | ©cmc/mN·m−1 |
|---|---|---|
| CTAB | 0.84 | 33.0 |
| CTAB + 3b | 0.57 | 32.4 |
| CTAB + 3c | 0.057 | 22.0 |
| CTAB + 3f | 0.10 | 20.8 |
| CTAB + 3g | 0.40 | 30.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.T.; Lobo, L.; Oliveira, I.S.; Gomes, J.; Teixeira, C.; Nogueira, F.; Marques, E.F.; Ferraz, R.; Gomes, P. Building on Surface-Active Ionic Liquids for the Rescuing of the Antimalarial Drug Chloroquine. Int. J. Mol. Sci. 2020, 21, 5334. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155334
Silva AT, Lobo L, Oliveira IS, Gomes J, Teixeira C, Nogueira F, Marques EF, Ferraz R, Gomes P. Building on Surface-Active Ionic Liquids for the Rescuing of the Antimalarial Drug Chloroquine. International Journal of Molecular Sciences. 2020; 21(15):5334. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155334
Chicago/Turabian StyleSilva, Ana Teresa, Lis Lobo, Isabel S. Oliveira, Joana Gomes, Cátia Teixeira, Fátima Nogueira, Eduardo F. Marques, Ricardo Ferraz, and Paula Gomes. 2020. "Building on Surface-Active Ionic Liquids for the Rescuing of the Antimalarial Drug Chloroquine" International Journal of Molecular Sciences 21, no. 15: 5334. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155334