Influence of Hypoxic and Hyperoxic Preconditioning on Endothelial Function in a Model of Myocardial Ischemia-Reperfusion Injury with Cardiopulmonary Bypass (Experimental Study)
Abstract
:1. Introduction
2. Results
2.1. Hemodynamic Data During the Experiment
2.2. Metabolic State During Experiment
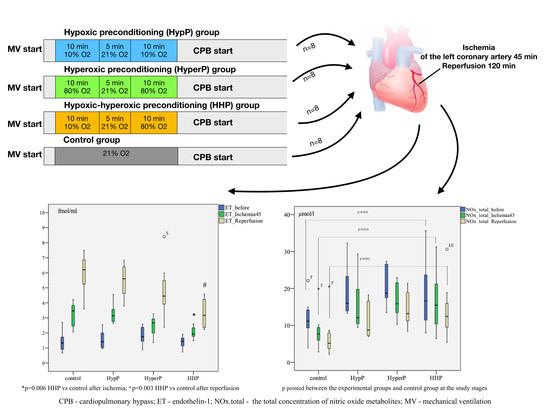
2.3. The Dynamics of Endothelin-1 Concentration in Blood Plasma During Experiment
2.4. Nitrite Production
2.5. Nitrate Production
2.6. Total Concentration of Nitric Oxide Metabolites
2.7. ADMA Production
2.8. Morphology
2.9. Morphometry
3. Discussion
4. Materials and Methods
4.1. Design
4.2. Animals and Experimental Procedures
4.3. Anaesthesia and Monitoring
4.4. Samples
4.5. Cardiopulmonary Bypass Technique
4.6. Ischemia-Reperfusion Protocol
4.7. Endothelial Function Markers
4.8. Myocardium Morphology and Morphometry
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NO | Nitric oxide |
| ET-1 | Endothelin-1 |
| ROS | Reactive oxygen species |
| CABG | Coronary artery bypass grafting |
| CPB | Cardiopulmonary bypass |
| ADMA | Asymmetric dimethylarginine |
| MV | Mechanical ventilation |
| HypP | Hypoxic preconditioning |
| HyperP | Hyperoxic preconditioning |
| HHP | Hypoxic-hyperoxic preconditioning |
| IRI | Ischemia-reperfusion injury |
| NO2− | Endogenous nitrite |
| NO3− | Endogenous nitrate |
| NOx | total total concentration of nitric oxide metabolites |
References
- Deng, Q.W.; Xia, Z.Q.; Qiu, Y.X.; Wu, Y.; Liu, J.X.; Li, C.; Liu, K.X. Clinical benefits of aortic cross-clamping versus limb remote ischemic preconditioning in coronary artery bypass grafting with cardiopulmonary bypass: A meta-analysis of randomized controlled trials. J. Surg. Res. 2015, 193, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Pitsis, A.A.; Rudiger, A.; Toller, W.; Longrois, D.; Ricksten, S.E.; Bobek, I.; De Hert, S.; Wieselthaler, G.; Schirmer, U.; et al. Clinical review: Practical recommendations on the management of perioperative heart failure in cardiac surgery. Crit. Care 2010, 14, 201. [Google Scholar] [CrossRef] [Green Version]
- Fedoruk, L.M.; Kron, I.L. An unusual case of cardiac dysfunction after left ventricular reconstruction. J. Cardiothorac. Surg. 2006, 1, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plicner, D.; Stoliński, J.; Wąsowicz, M.; Gawęda, B.; Hymczak, H.; Kapelak, B.; Drwiła, R.; Undas, A. Preoperative values of inflammatory markers predict clinical outcomes in patients after CABG, regardless of the use of cardiopulmonary bypass. Indian Heart J. 2016, 68 (Suppl. S3), S10–S15. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; van Royen, N.; Schulz, R.; Heusch, G. The Coronary Circulation in Acute Myocardial Ischaemia/Reperfusion Injury: A Target for Cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Barba, I.; Inserte, J. Twenty-five years of preconditioning: Are we ready for ischemia? From coronary occlusion to systems biology and back. Cardiovasc. Res. 2011, 91, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.M.; Yokoyama, T.; Horikawa, Y.; Roth, D.M.; Patel, H.H. Reactive oxygen species trigger ischemic and pharmacological postconditioning: In vivo and in vitro characterization. Life Sci. 2007, 81, 1223–1227. [Google Scholar] [CrossRef] [Green Version]
- Crestanello, J.A.; Lingle, D.M.; Kamelgard, J.; Millili, J.; Whitman, G.J. Ischemic preconditioning decreases oxidative stress during reperfusion: A chemiluminescence study. J. Surg. Res. 1996, 65, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Krylatov, A.V.; Maslov, L.N.; Voronkov, N.S.; Boshchenko, A.A.; Popov, S.V.; Gomez, L.; Wang, H.; Jaggi, A.S.; Downey, J.M. Reactive oxygen species as intracellular signaling molecules in the cardiovascular system. Curr. Cardiol. Rev. 2018, 14, 290–300. [Google Scholar] [CrossRef]
- Garlid, A.O.; Jaburek, M.; Jacobs, J.P.; Garlid, K.D. Mitochondrial reactive oxygen species: Which ROS signals cardioprotection? Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H960–H968. [Google Scholar] [CrossRef] [Green Version]
- Sanada, S.; Komuro, I.; Kitakaze, M. Pathophysiology of myocardial reperfusion injury: Preconditioning, postconditioning, and translational aspects of protective measures. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1723–H1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; LaManna, J.C. Short-term hypoxic preconditioning improved survival following cardiac arrest and resuscitation in rats. Adv. Exp. Med. Biol. 2014, 812, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Di Venosa, N.; Moro, N.; Colantuono, G.; Paradies, V.; Tiravanti, E.; Federici, A.; Ruggiero, F.M.; Paradies, G. In vivo hyperoxic preconditioning protects against rat-heart ischemia/reperfusion injury by inhibiting mitochondrial permeability transition pore opening and cytochrome c release. Free Radic. Biol. Med. 2011, 50, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Tähepõld, P.; Ruusalepp, A.; Li, G.; Vaage, J.; Starkopf, J.; Valen, G. Cardioprotection by breathing hyperoxic gas-relation to oxygen concentration and exposure time in rats and mice. Eur. J. Cardiothorac. Surg. 2002, 6, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Mandel, I.A.; Podoksenov, A.Y.; Sukhodolo, I.V.; Podoksenov, Y.u.K.; Svirko, Y.u.S.; Kamenshchikov, N.O.; Mikheev, S.L.; Sementsov, A.S.; Rogovskaya, Y.u.V.; An, D.A.; et al. Myocardial protection against ischemic and reperfusion injuries (Experimental study). Bull. Exp. Biol. Med. 2017, 164, 21–25. [Google Scholar] [CrossRef]
- Parthasarathi, K.; Lipowsky, H.H. Capillary recruitment in response to tissue hypoxia and its dependence on red cell deformability. J. Am. Physiol. 1999, 277, H2145–H2157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Lee, J.W.; Bowser, J.L.; Neudecker, V.; Sridhar, S.; Eltzschig, H.K. Targeting Hypoxia Signaling for Perioperative Organ Injury. Anesth. Analg. 2018, 126, 308. [Google Scholar] [CrossRef]
- Maslov, L.N.; Lishmanov, Y.B.; Krylatov, A.V.; Sementsov, A.S.; Portnichenko, A.G.; Podoksenov, Y.K.; Khaliulin, I.G. Comparative analysis of early and delayed cardioprotective and antiarrhythmic efficacy of hypoxic preconditioning. Bull. Exp. Biol. Med. 2014, 156, 746–749. [Google Scholar] [CrossRef]
- Kopterides, P.; Kapetanakis, T.; Siempos, I.I.; Magkou, C.; Pelekanou, A.; Tsaganos, T.; Giamarellos-Bourboulis, E.; Roussos, C. Armaganidis A Short-term administration of a high oxygen concentration is not injurious in an ex-vivo rabbit model of ventilator-induced lung injury. Anesth. Analg. 2009, 108, 556–564. [Google Scholar] [CrossRef]
- Rocco, M.; D’Itri, L.; De Bels, D.; Corazza, F.; Balestra, C. The “normobaric oxygen paradox”: A new tool for the anesthetist? Minerva Anestesiol 2014, 80, 366–372. [Google Scholar]
- Baharvand, B.; Dehaj, M.E.; Foadaddini, M.; Rasoulian, B.; Poorkhalili, K.; Aghai, H.W.; Khoshbaten, A. Delay cardioprotective effects of hyperoxia preconditioning prolonged by intermittent exposure. J. Surg. Res. 2010, 160, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. Hyperoxia: A review of the risks and benefits in adult cardiac surgery. J. Extra-Corpor. Technol. 2012, 44, 241. [Google Scholar] [PubMed]
- Glazachev, O.; Kopylov, P.; Susta, D.; Dudnik, E.; Zagaynaya, E. Adaptations following an intermittent hypoxia-hyperoxia training in coronary artery disease patients: A controlled study. Clin. Cardiol. 2017, 40, 370–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Pascual, F.; Busnadiego, O.; Lagares, D.; Lamas, S. Role of endothelin in the cardiovascular system. Pharmacol. Res. 2011, 63, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Kolettis, T.M.; Barton, M.; Langleben, D.; Matsumura, Y. Endothelin in coronary artery disease and myocardial infarction. Cardiol. Rev. 2013, 21, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Dorman, B.H.; Kratz, J.M.; Multani, M.M.; Baron, R.; Farrar, E.; Walton, S.; Payne, K.; Ikonomiois, J.; Reeves, S.; Mukherjee, R.; et al. A prospective, randomized study of endothelin and postoperative recovery in off-pump versus conventional coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 2004, 18, 25–29. [Google Scholar] [CrossRef]
- Kamenshchikov, N.O.; Mandel, I.A.; Podoksenov, Y.K.; Svirko, Y.S.; Lomivorotov, V.V.; Mikheev, S.L.; Kozlov, B.N.; Shipulin, V.M.; Nenakhova, A.A.; Anfinogenova, Y.J. Nitric oxide provides myocardial protection when added to the cardiopulmonary bypass circuit during cardiac surgery: Randomized trial. J. Thorac. Cardiovasc. Surg. 2019, 157, 2328–2336. [Google Scholar] [CrossRef]
- Calvert, J.W.; Lefer, D.J. Myocardial protection by nitrite. Cardiovasc. Res. 2009, 83, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Bolli, R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: An overview of a decade of research. J. Mol. Cell. Cardiol. 2001, 33, 1897–1918. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, J.; Hoyle, C.H.V.; Burnstock, G. Nitric Oxide in Health and Disease. In Biomedical Research Topics (1) American Scientist; Cambridge University Press: Cambridge, UK, 1997; p. 363. ISBN 9780521559775. [Google Scholar]
- Schwedhelm, E.; Boger, R.H. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat. Rev. Nephrol. 2011, 7, 275–285. [Google Scholar] [CrossRef]
- Konya, H.; Miuchi, M.; Satani, K.; Matsutani, S.; Yano, Y.; Tsunoda, T.; Ikawa, T.; Matsuo, T.; Ochi, F.; Kusunoki, Y.; et al. Asymmetric dimethylarginine, a biomarker of cardiovascular complications in diabetes mellitus. World J. Exp. Med. 2015, 5, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Lanzarone, E.; Gelmini, F.; Fumero, A.; Carini, M.; Costantino, M.L.; Fumero, R.; Alfieri, O. Preservation of endothelium nitric oxide release during beating heart surgery with respect to continuous flow cardiopulmonary bypass. Perfusion 2010, 25, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Viaro, F.; Baldo, C.F.; Capellini, V.K.; Celotto, A.C.; Bassetto, S.; Rodrigues, A.J.; Evora, P.R. Plasma nitrate/nitrite (NOx) is not a useful biomarker to predict inherent cardiopulmonary bypass inflammatory response. J. Card. Surg. 2008, 23, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Cernacek, P.; Stewart, D.J.; Monge, J.-C.; Rouleau, J.-L. The endothelin system and its role in acute myocardial infarction. Can. J. Physiol. Pharmacol. 2003, 81, 598–606. [Google Scholar] [CrossRef]
- Brett, S.J.; Quinlan, G.J.; Mitchell, J.; Pepper, J.R.; Evans, T.W. Production of nitric oxide during surgery involving cardiopulmonary bypass. Crit. Care Med. 1998, 26, 272–278. [Google Scholar] [CrossRef]
- Lazaratos, S.; Kashimura, H.; Nakahara, A.; Fukutomi, H.; Osuga, T.; Goto, K. L-arginine and endogenous nitric oxide protect the gastric mucosa from endothelin-l-induced gastric ulcers in rats. J. Gastroenterol. 1995, 30, 578–584. [Google Scholar] [CrossRef]
- Cabigas, B.P.; Su, J.; Hutchins, W.; Shi, Y.; Schaefer, R.B.; Recinos, R.F.; Nilakantan, V.; Kindwall, E.; Niezgoda, J.A.; Baker, J.E.; et al. Hyperoxic and hyperbaric-induced cardioprotection: Role of nitric oxide synthase 3. Cardiovasc. Res. 2006, 72, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Stühlinger, M.C.; Conci, E.; Haubner, B.J.; Stocker, E.M.; Schwaighofer, J.; Cooke, J.P.; Tsao, P.S.; Pachinger, O.; Metzler, B. Asymmetric Dimethyl L-Arginine (ADMA) is a critical regulator of myocardial reperfusion injury. Cardiovasc. Res. 2007, 75, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Yellon, D.M.; Downey, J.M. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol. Rev. 2003, 83, 1113–1151. [Google Scholar] [CrossRef]
- Vignon-Zellweger, N.; Heiden, S.; Miyauchi, T.; Emoto, N. Endothelin and endothelin receptors in the renal and cardiovascular systems. Life Sci. 2012, 91, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Heusch, G. Molecular Basis of Cardioprotection Signal Transduction in Ischemic Pre-, Post-, and Remote Conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Schlüter, K.-D.; Schermuly, R.T.; Schulz, R. Cardioprotection in right heart failure. Br. J. Phamacol. 2020, 1–19. [Google Scholar] [CrossRef]
- Bibli, S.-I.; Andreadou, I.; Lazaris, E.; Zoga, A.; Varnavas, V.; Andreou, C.C.; Dagres, N.; Iliodromitis, E.K.; Kyriakides, Z.S. Myocardial Protection Provided by Chronic Skeletal Muscle Ischemia Is Not Further Enhanced by Ischemic Pre- or Postconditioning: Comparative Effects on Intracellular Signaling. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, H.J.F.; Schultz, M.J.; van der Voort, P.H.; deJonge, E.; van Westerloo, D.J. Bench-to-bedside review: The effects of hyperoxia during critical illness. Crit. Care 2015, 19, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandel, I.A.; Podoksenov, Y.K.; Mikheev, S.L.; Svirko, Y.u.S.; Sukhodolo, I.V.; Shipulin, V.M.; Kamenshchikov, N.O.; Yaroshetskiy, A.I.; Yavorovskiy, A.G. The effect of hypoxic-hyperoxic preconditioning on the development of postoperative complications and oxygen transport in coronary surgery with a cardiopulmonary bypass. J. Anaesthesiol. Reanimatol. 2018, 63, 38–45. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]


 ), preserved myofibrils; only a few pyknotic nuclei (
), preserved myofibrils; only a few pyknotic nuclei (  ); Ischemic area (IA) HHP group: hyperaemia, interstitial edema (
); Ischemic area (IA) HHP group: hyperaemia, interstitial edema (  ), perinuclear vacuolization (
), perinuclear vacuolization (  ), enlarged capillaries (
), enlarged capillaries (  );AR Control group: severe dystrophy (
);AR Control group: severe dystrophy (  ) of cardiomyocytes, loss of cross-striation (
) of cardiomyocytes, loss of cross-striation (  ), partial degradation of myofibrils; massive cariopicnose (
), partial degradation of myofibrils; massive cariopicnose (  ); IA Control group: hyperaemia, interstitial edema (
); IA Control group: hyperaemia, interstitial edema (  ), perinuclear vacuolization (
), perinuclear vacuolization (  ), partial degradation of myofibrils, enlarged capillaries (
), partial degradation of myofibrils, enlarged capillaries (  ), local haemorrhages; AR HyperP group: dystrophy of cardiomyocytes, preserved cross-striation and myofibrils (
), local haemorrhages; AR HyperP group: dystrophy of cardiomyocytes, preserved cross-striation and myofibrils (  ); a few pyknotic nuclei (
); a few pyknotic nuclei (  ), enlarged capillaries (
), enlarged capillaries (  ); IA HyperP group: hyperaemia, interstitial edema (
); IA HyperP group: hyperaemia, interstitial edema (  ), perinuclear vacuolization (
), perinuclear vacuolization (  ), enlarged capillaries (
), enlarged capillaries (  ), dystrophy (
), dystrophy (  ) of cardiomyocytes; AR HypP group: dystrophy of cardiomyocytes (
) of cardiomyocytes; AR HypP group: dystrophy of cardiomyocytes (  ), loss of cross-striation (
), loss of cross-striation (  ), partial degradation of myofibrils; cariopicnose (
), partial degradation of myofibrils; cariopicnose (  ), interstitial edema (
), interstitial edema (  ); IA HypP group: hyperaemia, interstitial edema (
); IA HypP group: hyperaemia, interstitial edema (  ), partial degradation of myofibrils, enlarged capillaries (
), partial degradation of myofibrils, enlarged capillaries (  ), loss of cross-striation (
), loss of cross-striation (  ), local haemorrhages; 200×, H&E.
), local haemorrhages; 200×, H&E.
 ), preserved myofibrils; only a few pyknotic nuclei (
), preserved myofibrils; only a few pyknotic nuclei (  ); Ischemic area (IA) HHP group: hyperaemia, interstitial edema (
); Ischemic area (IA) HHP group: hyperaemia, interstitial edema (  ), perinuclear vacuolization (
), perinuclear vacuolization (  ), enlarged capillaries (
), enlarged capillaries (  );AR Control group: severe dystrophy (
);AR Control group: severe dystrophy (  ) of cardiomyocytes, loss of cross-striation (
) of cardiomyocytes, loss of cross-striation (  ), partial degradation of myofibrils; massive cariopicnose (
), partial degradation of myofibrils; massive cariopicnose (  ); IA Control group: hyperaemia, interstitial edema (
); IA Control group: hyperaemia, interstitial edema (  ), perinuclear vacuolization (
), perinuclear vacuolization (  ), partial degradation of myofibrils, enlarged capillaries (
), partial degradation of myofibrils, enlarged capillaries (  ), local haemorrhages; AR HyperP group: dystrophy of cardiomyocytes, preserved cross-striation and myofibrils (
), local haemorrhages; AR HyperP group: dystrophy of cardiomyocytes, preserved cross-striation and myofibrils (  ); a few pyknotic nuclei (
); a few pyknotic nuclei (  ), enlarged capillaries (
), enlarged capillaries (  ); IA HyperP group: hyperaemia, interstitial edema (
); IA HyperP group: hyperaemia, interstitial edema (  ), perinuclear vacuolization (
), perinuclear vacuolization (  ), enlarged capillaries (
), enlarged capillaries (  ), dystrophy (
), dystrophy (  ) of cardiomyocytes; AR HypP group: dystrophy of cardiomyocytes (
) of cardiomyocytes; AR HypP group: dystrophy of cardiomyocytes (  ), loss of cross-striation (
), loss of cross-striation (  ), partial degradation of myofibrils; cariopicnose (
), partial degradation of myofibrils; cariopicnose (  ), interstitial edema (
), interstitial edema (  ); IA HypP group: hyperaemia, interstitial edema (
); IA HypP group: hyperaemia, interstitial edema (  ), partial degradation of myofibrils, enlarged capillaries (
), partial degradation of myofibrils, enlarged capillaries (  ), loss of cross-striation (
), loss of cross-striation (  ), local haemorrhages; 200×, H&E.
), local haemorrhages; 200×, H&E.
 ), the capsule of the glomerulus (
), the capsule of the glomerulus (  ) is moderately expanded, 200×, H&E; Control group (B): marked edema (
) is moderately expanded, 200×, H&E; Control group (B): marked edema (  ), enlarged capillaries (
), enlarged capillaries (  ) of cortical and medullar substances, the capsule of the glomerulus (
) of cortical and medullar substances, the capsule of the glomerulus (  ) is moderately expanded, 200×, H&E; HyperP group (C): the nuclei of the podocytes are normal, the capsule of the glomerulus (
) is moderately expanded, 200×, H&E; HyperP group (C): the nuclei of the podocytes are normal, the capsule of the glomerulus (  ) is moderately expanded, a moderate amount of red blood cells in the capillaries. 400×, H&E; HypP group (D): enlarged capillaries (
) is moderately expanded, a moderate amount of red blood cells in the capillaries. 400×, H&E; HypP group (D): enlarged capillaries (  ) of cortical substance, edema (
) of cortical substance, edema (  ), destructive changes in cells. 400×, H&E.
), destructive changes in cells. 400×, H&E.
 ), the capsule of the glomerulus (
), the capsule of the glomerulus (  ) is moderately expanded, 200×, H&E; Control group (B): marked edema (
) is moderately expanded, 200×, H&E; Control group (B): marked edema (  ), enlarged capillaries (
), enlarged capillaries (  ) of cortical and medullar substances, the capsule of the glomerulus (
) of cortical and medullar substances, the capsule of the glomerulus (  ) is moderately expanded, 200×, H&E; HyperP group (C): the nuclei of the podocytes are normal, the capsule of the glomerulus (
) is moderately expanded, 200×, H&E; HyperP group (C): the nuclei of the podocytes are normal, the capsule of the glomerulus (  ) is moderately expanded, a moderate amount of red blood cells in the capillaries. 400×, H&E; HypP group (D): enlarged capillaries (
) is moderately expanded, a moderate amount of red blood cells in the capillaries. 400×, H&E; HypP group (D): enlarged capillaries (  ) of cortical substance, edema (
) of cortical substance, edema (  ), destructive changes in cells. 400×, H&E.
), destructive changes in cells. 400×, H&E.
 ), 670x, H&E; Control group (B): a large number of intraepithelial lymphocytes (
), 670x, H&E; Control group (B): a large number of intraepithelial lymphocytes (  ), 400×, H&E; HyperP group (C): intestinal villus edema (
), 400×, H&E; HyperP group (C): intestinal villus edema (  ), 400×, H&E; HypP group (D): intestinal villi edema (
), 400×, H&E; HypP group (D): intestinal villi edema (  ), a large number of intraepithelial lymphocytes (
), a large number of intraepithelial lymphocytes (  ), 400×, H&E.
), 400×, H&E.
 ), 670x, H&E; Control group (B): a large number of intraepithelial lymphocytes (
), 670x, H&E; Control group (B): a large number of intraepithelial lymphocytes (  ), 400×, H&E; HyperP group (C): intestinal villus edema (
), 400×, H&E; HyperP group (C): intestinal villus edema (  ), 400×, H&E; HypP group (D): intestinal villi edema (
), 400×, H&E; HypP group (D): intestinal villi edema (  ), a large number of intraepithelial lymphocytes (
), a large number of intraepithelial lymphocytes (  ), 400×, H&E.
), 400×, H&E.
 ) of the interalveolar capillaries, 400×, H&E; Control group (B): enlarged capillaries (
) of the interalveolar capillaries, 400×, H&E; Control group (B): enlarged capillaries (  ), filled with erythrocytes, 400×, H&E; HyperP group (C): moderate hyperemia (
), filled with erythrocytes, 400×, H&E; HyperP group (C): moderate hyperemia (  ) of the interalveolar capillaries, 400×, H&E; HypP group (D): edema (
) of the interalveolar capillaries, 400×, H&E; HypP group (D): edema (  ) of the interalveolar septa, dystrophy of alveoles, atelectasis (
) of the interalveolar septa, dystrophy of alveoles, atelectasis (  ), 400×, H&E.
), 400×, H&E.
 ) of the interalveolar capillaries, 400×, H&E; Control group (B): enlarged capillaries (
) of the interalveolar capillaries, 400×, H&E; Control group (B): enlarged capillaries (  ), filled with erythrocytes, 400×, H&E; HyperP group (C): moderate hyperemia (
), filled with erythrocytes, 400×, H&E; HyperP group (C): moderate hyperemia (  ) of the interalveolar capillaries, 400×, H&E; HypP group (D): edema (
) of the interalveolar capillaries, 400×, H&E; HypP group (D): edema (  ) of the interalveolar septa, dystrophy of alveoles, atelectasis (
) of the interalveolar septa, dystrophy of alveoles, atelectasis (  ), 400×, H&E.
), 400×, H&E.


| Data | Hypoxic Preconditioning, n = 8 | Hyperoxic Preconditioning, n = 8 | Hypoxic-hyperoxic Preconditioning, n = 8 | Control, n = 8 | p |
|---|---|---|---|---|---|
| paO2, mmHg | 116 [108.8; 187] | 187 [121.0; 196.8] | 142 [125.8; 175.8] | 187 [114; 196.8] | 0.455 |
| pvO2, mmHg | 42 [39.0; 48.8] | 42 [39.5; 44.3] | 39 [38.3; 48.8] | 40.5 [39.0; 41.8] | 0.760 |
| dPv-aCO2 /Ca-vO2 | 0.8 [0.7; 0.9] | 1.1 [0.9; 1.2] | 0.9 [0.8; 1.1] | 0.9 [0.8; 1.1] | 0.249 |
| SaO2 | 98 [96.0; 98.8] | 98.5 [97.3; 99.0] | 97 [96.0; 98.0] | 98.5 [97.3; 99.8] | 0.194 |
| SvO2 | 54.5 [48; 72] | 59 [55.5; 65.0] | 55 [49.0; 68.3] | 56 [48.0; 60.8] | 0.597 |
| IEO2 | 43.8 [26.7; 50.4] | 40.4 [34.5; 44.1] | 43.3 [30.2; 50.2] | 43.1 [37.3; 51.4] | 0.704 |
| Glu, mmol/L | 7.5 [6.0; 8.0] | 6 [5.0; 6.8] | 7 [6.0; 7.8] | 5.5 [5.0; 6.8] | 0.075 |
| Lac, mmol/L | 4 [3.1; 5.0] | 3.5 [3.0; 5.0] | 3.2 [3.0; 4.6] | 4.5 [3.3; 5.0] | 0.533 |
| Data | Hypoxic Preconditioning, n = 8 | Hyperoxic Preconditioning, n = 8 | Hypoxic-Hyperoxic Preconditioning, n = 8 | Control, n = 8 | p |
|---|---|---|---|---|---|
| Physiological variable | |||||
| Oesophagus temperature, °C | 36.5 [36.4; 37.0] | 37 [36.7; 37.2] | 36.9 [36.6; 37.0] | 37.1 [36.6; 37.4] | 0.181 |
| Hemodynamic variables | |||||
| Heart rate, beats/min | 180.5 [167.8; 187.8] | 179 [176.5; 186.3] | 191 [186.5; 202.0] | 190.5 [171.8; 193.0] | 0.131 |
| Mean arterial pressure, mmHg | 69.5 [68.3; 72.5] | 65.5 [60.0; 70.3] | 70 [62.5; 73.3] | 68 [65.0; 71.5] | 0.474 |
| Hemoglobin, g/L | 91.5 [89.3; 109] | 100.5 [98.3; 108.3] | 96 [90.5; 102.5] | 97.5 [91.8; 103.8] | 0.517 |
| Diuresis, mL/kg/h | 1.6 [1.5; 1.7] | 1.35 [1.05; 1.63] | 1.3 [1.02; 1.68] | 1.45 [1.23; 1.58] | 0.195 |
| Data | Hypoxic Preconditioning, n = 8 | Hyperoxic Preconditioning, n = 8 | Hypoxic-hyperoxic Preconditioning, n = 8 | |
|---|---|---|---|---|
| 20 min | 20 min | Hypoxic Phase, 10 min | Hyperoxic Phase, 10 min | |
| paO2, mmHg | 50.5 [49; 52.8] * | 348 [317.5; 392.5] * | 52 [48.3; 58.8] * | 340 [310; 375] * |
| pvv2, mmHg | 39 [37.3; 40] | 48 [46; 50.8] | 37 [37; 39.5] | 47.5 [44.3; 52] |
| dPv-aCO2 /Ca-vO2 | 0.8 [0.7; 0.9] | 1.1 [0.9; 1.2] | 1.0 [0.8; 1.1] | 1.1 [0.9; 1.2] |
| SaO2 | 82.5 [77; 85]* | 99 [99; 100] | 82 [77.3; 85.5]* | 99 [99; 99] |
| SvO2 | 50 [49; 52.3] | 64.5 [56.3; 68.8] | 50 [48.3; 53.3] | 59 [56.5; 67] |
| IEO2 | 38.9 [33.4; 42.1] | 35.2 [31.3; 43.2] | 38.9 [33.4; 42.3] | 40.4 [31.8; 42.9] |
| Glu, mmol/L | 6 [5.3; 6.8] | 6 [4; 7] | 4.5 [4; 6.8] | 7 [5.3; 8] |
| Lac, mmol/L | 3 [3; 3.8] | 5 [4.3; 6] | 4 [3; 4.8] | 5 [4; 6] |
| Parameter | HypP, n = 8 | HyperP, n = 8 | HHP, n = 8 | Control, n = 8 | p # between Groups |
|---|---|---|---|---|---|
| Changes in nitrite concentration | |||||
| NO2.endo, before ischemia, µmol/L | 0.947 [0.806; 1.187] | 0.822 [0.585; 0.976] | 1.095 [0.906; 1.331] | 0.442 [0.366; 0.573] | 0.010 |
| NO2.endo, after ischemia, µmol/L | 0.732 [0.572; 0.857] | 0.627 [0.555; 0.811] | 0.882 [0.631; 1.042] | 0.266 [0.217; 0.345] | 0.005 |
| p * in comparison with the initial value | p = 0.012 | p = 0.069 | p = 0.012 | p = 0.012 | |
| NO2.endo, after reperfusin, µmol/L | 0.511 [0.378; 0.640] | 0.505 [0.409; 0.633] | 0.706 [0.590; 0.838] | 0.203 [0.180; 0.319] | 0.002 |
| p * in comparison with the initial value | p = 0.012 | p = 0.161 | p = 0.012 | p = 0.012 | |
| Changes in nitrate concentration | |||||
| NO3.endo, before ischemia, µmol/L | 14.803 [12.756; 22.903] | 18.033 [16.247; 25.839] | 13.808 [10.223; 24.836] | 10.732 [8.740; 14.317] | 0.045 |
| NO3.endo, after ischemia, µmol/L | 11.451 [9.595; 19.366] | 15.214 [12.764; 21.260] | 13.995 [9.019; 22.961] | 7.399 [5.570; 9.664] | 0.027 |
| p * in comparison with the initial value | p = 0.012 | p = 0.012 | p = 0.484 | p = 0.012 | |
| NO3.endo, after reperfusion, µmol/L | 8.177 [6.727; 16.170] | 12.202 [10.187; 18.981] | 10.832 [8.683; 16.600] | 4.938 [3.526; 8.168] | 0.026 |
| p * in comparison with the initial value | p = 0.012 | p = 0.012 | p = 0.069 | p = 0.012 | |
| Changes in ADMA concentration | |||||
| ADMA, before ischemia, µmol/L | 1.659 [1.400; 2.593] | 1.692 [1.567; 1.997] | 1.617 [1.449; 1.944] | 1.746 [1.255; 2.147] | 0.827 |
| ADMA, after ischemia, µmol/L | 1.62 [1.50; 2.395] | 1.781 [1.564; 1.985] | 1.684 [1.328; 1.893] | 2.310 [2.092; 2.732] | 0.036 |
| p * in comparison with the initial value | p = 0.575 | p = 0.575 | p = 0.484 | p = 0.036 | |
| ADMA, after reperfusion, µmol/L | 1.727 [1.680; 2.322] | 1.79 [1.571; 1.890] | 1.803[1.450; 1.956] | 2.325 [1.864; 2.678] | 0.084 |
| p * in comparison with the initial value | p = 0.779 | p = 0.263 | p = 0.674 | p = 0.050 | |
| Ischemic Area, Control Group, n = 8 | Ischemic Area, HHP Group, n = 8 | Area at Risk, Control Group, n = 8 | Area at Risk, HHP Group, n = 8 | |
|---|---|---|---|---|
| Cardiomyocyte, μm3/μm3 | 0.687 [0.632; 0.742] | 0.722 [0.643; 0.758] | 0.603 [0.550; 0.694] | 0.718 [0.679; 0.782] * |
| Nuclei, μm3/μm3 | 0.057 [0.029; 0.083] | 0.046 [0.027; 0.080] | 0.035 [0.027; 0.045] | 0.052 [0.024; 0.068] |
| Perinuclear vacuolization, μm3/μm3 | 0.047 [0.028; 0.068] | 0.027 [0.011; 0.054] * | 0.046[0.019; 0.071] | 0.025 [0.010; 0.050] * |
| Interstitial oedema, μm3/μm3 | 0.128 [0.083; 0.168] | 0.102 [0.067; 0.152] | 0.17 [0.135; 0.232] | 0.091 [0.062; 0.129] * |
| Vessels,μm3/μm3 | 0.057 [0.025; 0.101] | 0.076 [0.035; 0.103] | 0.114 [0.093; 0.145] | 0.067 [0.035; 0.103] * |
| Cardiomyocytes | Ischemic Area, Control Group, n = 8 | Ischemic Area, HHP Group, n = 8 | Area at Risk, Control Group, n = 8 | Area at Risk, HHP Group, n = 8 |
|---|---|---|---|---|
| Ischemic, μm3/μm3 | 0.432 [0.313; 0.533] | 0.329 [0.240; 0.438] * | 0.481 [0.346; 0.577] | 0.268 [0.112; 0.361] * |
| Non-ischemic, μm3/μm3 | 0.568 [0.467; 0.687] | 0.671 [0.562; 0.760] * | 0.519 [0.423; 0.654] | 0.732 [0.639; 0.888] * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandel, I.A.; Podoksenov, Y.K.; Suhodolo, I.V.; An, D.A.; Mikheev, S.L.; Podoksenov, A.Y.; Svirko, Y.S.; Gusakova, A.M.; Shipulin, V.M.; Yavorovskiy, A.G. Influence of Hypoxic and Hyperoxic Preconditioning on Endothelial Function in a Model of Myocardial Ischemia-Reperfusion Injury with Cardiopulmonary Bypass (Experimental Study). Int. J. Mol. Sci. 2020, 21, 5336. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155336
Mandel IA, Podoksenov YK, Suhodolo IV, An DA, Mikheev SL, Podoksenov AY, Svirko YS, Gusakova AM, Shipulin VM, Yavorovskiy AG. Influence of Hypoxic and Hyperoxic Preconditioning on Endothelial Function in a Model of Myocardial Ischemia-Reperfusion Injury with Cardiopulmonary Bypass (Experimental Study). International Journal of Molecular Sciences. 2020; 21(15):5336. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155336
Chicago/Turabian StyleMandel, Irina A., Yuri K. Podoksenov, Irina V. Suhodolo, Darya A. An, Sergey L. Mikheev, Andrey Yu. Podoksenov, Yulia S. Svirko, Anna M. Gusakova, Vladimir M. Shipulin, and Andrey G. Yavorovskiy. 2020. "Influence of Hypoxic and Hyperoxic Preconditioning on Endothelial Function in a Model of Myocardial Ischemia-Reperfusion Injury with Cardiopulmonary Bypass (Experimental Study)" International Journal of Molecular Sciences 21, no. 15: 5336. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155336