Preconditioned and Genetically Modified Stem Cells for Myocardial Infarction Treatment
Abstract
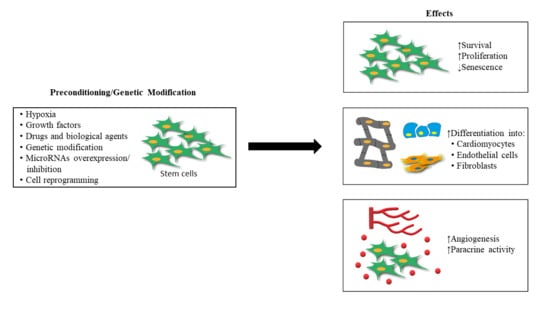
:1. Introduction
2. Stem Cell Preconditioning for MI Treatment
2.1. Hypoxic Preconditioning of Stem Cells
2.2. Preconditioning with Growth Factors
2.3. Preconditioning with Drugs and Biological Agents
3. Genetically Modified Stem Cell for MI Treatment
3.1. Genetic Modification
3.2. Overexpression or Inhibition of Specific MicroRNAs
3.3. Cell Reprogramming and Stem Cell Differentiation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, E139–E596. [Google Scholar] [PubMed]
- McClellan, M.; Brown, N.; Califf, R.M.; Warner, J.J. Call to action: Urgent challenges in cardiovascular disease: A presidential advisory from the American Heart Association. Circulation 2019, 139, e44–e54. [Google Scholar] [CrossRef]
- American Heart Association. Cardiovascular Disease: A Costly Burden for America Projections through 2035; Heart.org: Washington, DC, USA, 2017. [Google Scholar]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from ischemic heart disease: Analysis of data from the World Health Organization and coronary artery disease risk factors From NCD Risk Factor Collaboration. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef]
- Cambria, E.; Pasqualini, F.S.; Wolint, P.; Günter, J.; Steiger, J.; Bopp, A.; Hoerstrup, S.P.; Emmert, M.Y. Translational cardiac stem cell therapy: Advancing from first-generation to next-generation cell types. NPJ Regen. Med. 2017, 2, 17. [Google Scholar] [CrossRef]
- Awada, H.K.; Hwang, M.P.; Wang, Y. Towards comprehensive cardiac repair and regeneration after myocardial infarction: Aspects to consider and proteins to deliver. Biomaterials 2016, 82, 94–112. [Google Scholar] [CrossRef] [Green Version]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Chen, W.C.; Mansurov, N.; Issabekova, A.; Zhakupova, J. Role of the immune system in cardiac tissue damage and repair following myocardial infarction. Inflamm. Res. 2017, 66, 739–751. [Google Scholar] [CrossRef]
- Anderson, J.L.; Morrow, D.A. Acute myocardial infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [Green Version]
- Kittleson, M.M.; Kobashigawa, J.A. Cardiac transplantation: Current outcomes and contemporary controversies. JACC Heart Fail. 2017, 5, 857–868. [Google Scholar]
- Higuchi, A.; Ku, N.J.; Tseng, Y.C.; Pan, C.H.; Li, H.F.; Kumar, S.S.; Ling, Q.D.; Chang, Y.; Alarfaj, A.A.; Munusamy, M.A.; et al. Stem cell therapies for myocardial infarction in clinical trials: Bioengineering and biomaterial aspects. Lab. Investig. J. Tech. Methods Pathol. 2017, 97, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.; Lemcke, H.; David, R. Stem cell therapy in heart diseases–cell types, mechanisms and improvement strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef] [PubMed]
- Smagul, S.; Kim, Y.; Smagulova, A.; Raziyeva, K.; Nurkesh, A.; Saparov, A. Biomaterials Loaded with Growth Factors/Cytokines and Stem Cells for Cardiac Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 5952. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Wu, R.; Hu, X.; Wang, J.A. Concise review: Optimized strategies for stem cell-based therapy in myocardial repair: Clinical translatability and potential limitation. Stem Cells 2018, 36, 482–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, A.; Fernández-Avilés, F.; Dimmeler, S.; Hauskeller, C.; Janssens, S.; Menasche, P.; Wojakowski, W.; Martin, J.F.; Zeiher, A.; BAMI Investigators. The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: Update 2016. Eur. Heart J. 2017, 38, 2930–2935. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: Final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail. 2020, 22, 884–892. [Google Scholar] [CrossRef]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madonna, R.; Van Laake, L.W.; Davidson, S.M.; Engel, F.B.; Hausenloy, D.J.; Lecour, S.; Leor, J.; Perrino, C.; Schulz, R.; Ytrehus, K.; et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016, 37, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K.; Neofytou, E.; Rhee, J.-W.; Wu, J.C. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: A review. JAMA Cardiol. 2016, 1, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T. Cardiomyocyte regeneration: A consensus statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef]
- Shafiq, M.; Jung, Y.; Kim, S.H. Insight on stem cell preconditioning and instructive biomaterials to enhance cell adhesion, retention, and engraftment for tissue repair. Biomaterials 2016, 90, 85–115. [Google Scholar] [CrossRef]
- Kim, J.; Shapiro, L.; Flynn, A. The clinical application of mesenchymal stem cells and cardiac stem cells as a therapy for cardiovascular disease. Pharmacol. Ther. 2015, 151, 8–15. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Zhang, X.; Liu, Y.; Chen, J.; Hu, B.; Song, J.; Zhang, Y. Strategies to optimize adult stem cell therapy for tissue regeneration. Int. J. Mol. Sci. 2016, 17, 982. [Google Scholar] [CrossRef] [Green Version]
- Karpov, A.A.; Udalova, D.V.; Pliss, M.G.; Galagudza, M.M. Can the outcomes of mesenchymal stem cell-based therapy for myocardial infarction be improved? Providing weapons and armour to cells. Cell Prolif. 2017, 50, e12316. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [Green Version]
- Ezquer, F.E.; Ezquer, M.E.; Vicencio, J.M.; Calligaris, S.D. Two complementary strategies to improve cell engraftment in mesenchymal stem cell-based therapy: Increasing transplanted cell resistance and increasing tissue receptivity. Cell Adhes. Migr. 2017, 11, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef]
- Braunwald, E. Cell-based therapy in cardiac regeneration: An overview. Circ. Res. 2018, 123, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Broughton, K.M.; Sussman, M.A. Enhancement strategies for cardiac regenerative cell therapy: Focus on adult stem cells. Circ. Res. 2018, 123, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, L.; Zeng, C.; Wang, W.E. Functionally improved mesenchymal stem cells to better treat myocardial infarction. Stem Cells Int. 2018, 2018, 7045245. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, H.; Voronina, N.; Steinhoff, G.; David, R. Recent progress in stem cell modification for cardiac regeneration. Stem Cells Int. 2018, 2018, 1909346. [Google Scholar] [CrossRef]
- Lennon, D.P.; Edmison, J.M.; Caplan, A.I. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: Effects on in vitro and in vivo osteochondrogenesis. J. Cell. Physiol. 2001, 187, 345–355. [Google Scholar] [CrossRef]
- Fehrer, C.; Brunauer, R.; Laschober, G.; Unterluggauer, H.; Reitinger, S.; Kloss, F.; Gülly, C.; Gaßner, R.; Lepperdinger, G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 2007, 6, 745–757. [Google Scholar]
- Rosová, I.; Dao, M.; Capoccia, B.; Link, D.; Nolta, J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008, 26, 2173–2182. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.Y.; Chun, S.Y.; Ha, Y.-S.; Kim, D.H.; Kim, J.; Song, P.H.; Kim, H.T.; Yoo, E.S.; Kim, B.S.; Kwon, T.G. Hypoxia enhances cell properties of human mesenchymal stem cells. Tissue Eng. Regen. Med. 2017, 14, 595–604. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, F.; Xue, E.; Huang, L.; Yan, P.; Pan, X.; Zhou, Y. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1α in injured neuronal cells derived exosomes culture system. Cell Death Dis. 2019, 10, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, S.H.; Bendigo, J.R.; Tobias, J.W.; Dodge, G.R.; Malhotra, N.R.; Mauck, R.L.; Smith, L.J. Hypoxic Preconditioning Enhances Bone Marrow–Derived Mesenchymal Stem Cell Survival in a Low Oxygen and Nutrient-Limited 3D Microenvironment. Cartilage 2019, 1947603519841675. [Google Scholar] [CrossRef] [PubMed]
- Wakai, T.; Narasimhan, P.; Sakata, H.; Wang, E.; Yoshioka, H.; Kinouchi, H.; Chan, P.H. Hypoxic preconditioning enhances neural stem cell transplantation therapy after intracerebral hemorrhage in mice. J. Cereb. Blood Flow Metab. 2016, 36, 2134–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheirandish, M.; Gavgani, S.P.; Samiee, S. The effect of hypoxia preconditioning on the neural and stemness genes expression profiling in human umbilical cord blood mesenchymal stem cells. Transfus. Apher. Sci. 2017, 56, 392–399. [Google Scholar] [CrossRef]
- Sart, S.; Ma, T.; Li, Y. Preconditioning stem cells for in vivo delivery. BioRes. Open Access 2014, 3, 137–149. [Google Scholar] [CrossRef]
- Saparov, A.; Chen, C.W.; Beckman, S.A.; Wang, Y.; Huard, J. The role of antioxidation and immunomodulation in postnatal multipotent stem cell-mediated cardiac repair. Int. J. Mol. Sci. 2013, 14, 16258–16279. [Google Scholar] [CrossRef] [Green Version]
- Mansurov, N.; Chen, W.C.; Awada, H.; Huard, J.; Wang, Y.; Saparov, A. A controlled release system for simultaneous delivery of three human perivascular stem cell-derived factors for tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1164–e1172. [Google Scholar] [CrossRef]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.A.; Wei, L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.Z.; Xiao, L.; Zhao, L.; Qiu, L.J.; Ma, Z.X.; Xu, X.W.; Liu, H.Y.; Zhou, T.T.; Wang, X.Y.; Tang, L.; et al. Molecular Mechanism Underlying Hypoxic Preconditioning-Promoted Mitochondrial Translocation of DJ-1 in Hypoxia/Reoxygenation H9c2 Cells. Molecules 2019, 25, 71. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, X.; Maureira, P.; Falanga, A.; Marie, V.; Gauchotte, G.; Poussier, S.; Groubatch, F.; Marie, P.Y.; Tran, N. Permanently Hypoxic Cell Culture Yields Rat Bone Marrow Mesenchymal Cells with Higher Therapeutic Potential in the Treatment of Chronic Myocardial Infarction. Cell. Physiol. Biochem. 2017, 44, 1064–1077. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, C.; Shen, M.; Yang, M.; Jin, Z.; Ding, L.; Jiang, W.; Yang, J.; Chen, H.; Cao, F.; et al. Autophagy mediates the beneficial effect of hypoxic preconditioning on bone marrow mesenchymal stem cells for the therapy of myocardial infarction. Stem Cell Res. Ther. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.H.; Hausenloy, D.J. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, K.; Wang, J.; Liu, K.; Wu, G.; Li, Y.; Luo, L.; Zheng, Z.; Hu, D. Hypoxic preconditioning combined with curcumin promotes cell survival and mitochondrial quality of bone marrow mesenchymal stem cells, and accelerates cutaneous wound healing via PGC-1α/SIRT3/HIF-1α signaling. Free Radic. Biol. Med. 2020, 159, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Zhong, Z.; Wu, Y.; Zhao, J.; Wang, Y.; Cheng, H.; Kong, M.; Zhang, F.; Chen, Q.; et al. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity Without Remuscularization. Circ. Res. 2016, 118, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.; Spohn, G.; Baer, P.C. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Transfus. Med. Hemotherapy 2016, 43, 256–267. [Google Scholar] [CrossRef] [Green Version]
- Majka, M.; Sułkowski, M.; Badyra, B.; Musiałek, P. Concise Review: Mesenchymal Stem Cells in Cardiovascular Regeneration: Emerging Research Directions and Clinical Applications. Stem Cells Transl. Med. 2017, 6, 1859–1867. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, D.; Li, W.F.; Li, H.R.; Zhang, A.D.; Li, Z.C. Insulin-like growth factor 1 treatment of MSCs attenuates inflammation and cardiac dysfunction following MI. Inflammation 2014, 37, 2156–2163. [Google Scholar] [CrossRef]
- Zhang, G.W.; Gu, T.X.; Guan, X.Y.; Sun, X.J.; Qi, X.; Li, X.Y.; Wang, X.B.; Lv, F.; Yu, L.; Jiang, D.Q.; et al. HGF and IGF-1 promote protective effects of allogeneic BMSC transplantation in rabbit model of acute myocardial infarction. Cell Prolif. 2015, 48, 661–670. [Google Scholar] [CrossRef]
- Farzaneh, M.; Rahimi, F.; Alishahi, M.; Khoshnam, S.E. Paracrine Mechanisms Involved in Mesenchymal Stem Cell Differentiation into Cardiomyocytes. Curr. Stem Cell Res. Ther. 2019, 14, 9–13. [Google Scholar] [CrossRef]
- Ling, L.; Gu, S.; Cheng, Y.; Ding, L. bFGF promotes Sca-1+ cardiac stem cell migration through activation of the PI3K/Akt pathway. Mol. Med. Rep. 2018, 17, 2349–2356. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.M.; Harn, H.J.; Chiou, T.W.; Chuang, M.H.; Chen, C.H.; Lin, P.C.; Lin, S.Z. Targeting the pathway of GSK-3β/nerve growth factor to attenuate post-infarction arrhythmias by preconditioned adipose-derived stem cells. J. Mol. Cell. Cardiol. 2017, 104, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Harn, H.J.; Chiou, T.W.; Chuang, M.H.; Chen, C.H.; Chuang, C.H.; Lin, P.C.; Lin, S.Z. Preconditioned adipose-derived stem cells ameliorate cardiac fibrosis by regulating macrophage polarization in infarcted rat hearts through the PI3K/STAT3 pathway. Lab. Investig. J. Tech. Methods Pathol. 2019, 99, 634–647. [Google Scholar] [CrossRef]
- Yan, W.; Lin, C.; Guo, Y.; Chen, Y.; Du, Y.; Lau, W.B.; Xia, Y.; Zhang, F.; Su, R.; Gao, E.; et al. N-Cadherin Overexpression Mobilizes the Protective Effects of Mesenchymal Stromal Cells Against Ischemic Heart Injury Through a β-Catenin-Dependent Manner. Circ. Res. 2020, 126, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, F.; Ruozi, G.; Falcione, A.; Doimo, S.; Dal Ferro, M.; Lesizza, P.; Zentilin, L.; Banks, L.; Zacchigna, S.; Giacca, M. In vivo functional selection identifies cardiotrophin-1 as a cardiac engraftment factor for mesenchymal stromal cells. Circulation 2017, 136, 1509–1524. [Google Scholar] [PubMed]
- Deng, J.; Zhang, N.; Wang, Y.; Yang, C.; Wang, Y.; Xin, C.; Zhao, J.; Jin, Z.; Cao, F.; Zhang, Z. FNDC5/irisin improves the therapeutic efficacy of bone marrow-derived mesenchymal stem cells for myocardial infarction. Stem Cell Res. Ther. 2020, 11, 228. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Guan, J.; Qi, X.; Ding, H.; Yuan, H.; Xie, Z.; Chen, C.; Li, X.; Zhang, C.; Huang, Y. Dimethyloxaloylglycine Promotes the Angiogenic Activity of Mesenchymal Stem Cells Derived from iPSCs via Activation of the PI3K/Akt Pathway for Bone Regeneration. Int. J. Biol. Sci. 2016, 12, 639–652. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.B.; Wang, J.A.; Ji, X.Y.; Yu, S.P.; Wei, L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res. Ther. 2014, 5, 111. [Google Scholar] [CrossRef] [Green Version]
- Marzilli, M. Cardioprotective effects of trimetazidine: A review. Curr. Med Res. Opin. 2003, 19, 661–672. [Google Scholar] [CrossRef]
- Ma, N.; Bai, J.; Zhang, W.; Luo, H.; Zhang, X.; Liu, D.; Qiao, C. Trimetazidine protects against cardiac ischemia/reperfusion injury via effects on cardiac miRNA-21 expression, Akt and the Bcl-2/Bax pathway. Mol. Med. Rep. 2016, 14, 4216–4222. [Google Scholar] [CrossRef]
- Wisel, S.; Khan, M.; Kuppusamy, M.L.; Mohan, I.K.; Chacko, S.M.; Rivera, B.K.; Sun, B.C.; Hideg, K.; Kuppusamy, P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J. Pharmacol. Exp. Ther. 2009, 329, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, H.; Yang, Y.J.; Dong, Q.T.; Wang, T.J.; Qian, H.Y.; Li, N.; Wang, X.M.; Jin, C. Atorvastatin treatment improves the effects of mesenchymal stem cell transplantation on acute myocardial infarction: The role of the RhoA/ROCK/ERK pathway. Int. J. Cardiol. 2014, 176, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Qian, H.Y.; Huang, J.; Geng, Y.J.; Gao, R.L.; Dou, K.F.; Yang, G.S.; Li, J.J.; Shen, R.; He, Z.X.; et al. Atorvastatin treatment improves survival and effects of implanted mesenchymal stem cells in post-infarct swine hearts. Eur. Heart J. 2008, 29, 1578–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Yang, Y.J.; Qian, H.Y.; Li, Q.; Zhang, Q.; Li, X.D.; Dong, Q.T.; Xu, H.; Song, L.; Zhang, H. Intravenous administration of atorvastatin-pretreated mesenchymal stem cells improves cardiac performance after acute myocardial infarction: Role of CXCR4. Am. J. Transl. Res. 2015, 7, 1058–1070. [Google Scholar] [PubMed]
- Zhang, Z.; Li, S.; Cui, M.; Gao, X.; Sun, D.; Qin, X.; Narsinh, K.; Li, C.; Jia, H.; Li, C.; et al. Rosuvastatin enhances the therapeutic efficacy of adipose-derived mesenchymal stem cells for myocardial infarction via PI3K/Akt and MEK/ERK pathways. Basic Res. Cardiol. 2013, 108, 333. [Google Scholar] [CrossRef]
- Noort, W.A.; Feye, D.; Van Den Akker, F.; Stecher, D.; Chamuleau, S.A.; Sluijter, J.P.; Doevendans, P.A. Mesenchymal stromal cells to treat cardiovascular disease: Strategies to improve survival and therapeutic results. Panminerva Med. 2010, 52, 27–40. [Google Scholar]
- Haneef, K.; Ali, A.; Khan, I.; Naeem, N.; Jamall, S.; Salim, A. Role of interleukin-7 in fusion of rat bone marrow mesenchymal stem cells with cardiomyocytes in vitro and improvement of cardiac function in vivo. Cardiovasc. Ther. 2018, 36, e12479. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Li, J.; Yu, M.; Yang, J.; Zheng, M.; Zhang, J.; Sun, C.; Liang, H.; Liu, L. Transplantation of mesenchymal stem cells overexpressing IL10 attenuates cardiac impairments in rats with myocardial infarction. J. Cell. Physiol. 2018, 233, 587–595. [Google Scholar] [CrossRef]
- Chen, Y.; Zuo, J.; Chen, W.; Yang, Z.; Zhang, Y.; Hua, F.; Shao, L.; Li, J.; Chen, Y.; Yu, Y.; et al. The enhanced effect and underlying mechanisms of mesenchymal stem cells with IL-33 overexpression on myocardial infarction. Stem Cell Res. Ther. 2019, 10, 295. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhu, J.; Zhang, N.; Liu, Q.; Wang, Y.; Hu, X.; Chen, J.; Zhu, W.; Yu, H. GDF11 enhances therapeutic efficacy of mesenchymal stem cells for myocardial infarction via YME1L-mediated OPA1 processing. Stem Cells Transl. Med. 2020. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Zhang, Y.; Liang, X.; Ding, Y.; Xu, Y.; Fang, Z.; Zhang, F. Enhanced cell survival and paracrine effects of mesenchymal stem cells overexpressing hepatocyte growth factor promote cardioprotection in myocardial infarction. Exp. Cell Res. 2016, 344, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Liu, X.; Zheng, H.; Huang, X.; Wu, Y.; Huang, A.; Zhu, H.; Hu, Y.; Mai, W.; Huang, Y. IGF-1 enhances BMSC viability, migration, and anti-apoptosis in myocardial infarction via secreted frizzled-related protein 2 pathway. Stem Cell Res. Ther. 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, S.M.; Jafarzadeh-Esfehani, R.; Ghandehari, M.; Parizadeh, M.R.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Stem cell therapy: A novel approach for myocardial infarction. J. Cell. Physiol. 2019, 234, 16904–16912. [Google Scholar] [CrossRef] [PubMed]
- Khorraminejad-Shirazi, M.; Dorvash, M.; Estedlal, A.; Hoveidaei, A.H.; Mazloomrezaei, M.; Mosaddeghi, P. Aging: A cell source limiting factor in tissue engineering. World J. Stem Cells 2019, 11, 787–802. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; He, H.; Fan, B.; Deng, R.; Hong, Y.; Liang, X.; Zhao, H.; Li, X.; Zhang, F. Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging 2019, 11, 12641–12660. [Google Scholar] [CrossRef]
- Xia, W.; Hou, M. Macrophage migration inhibitory factor rescues mesenchymal stem cells from doxorubicin-induced senescence though the PI3K-Akt signaling pathway. Int. J. Mol. Med. 2018, 41, 1127–1137. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Xia, W.; Hou, M. Macrophage migration inhibitory factor serves a pivotal role in the regulation of radiation-induced cardiac senescencethrough rebalancing the microRNA-34a/sirtuin 1 signaling pathway. Int. J. Mol. Med. 2018, 42, 2849–2858. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef]
- Alijani-Ghazyani, Z.; Sabzevari, R.; Roushandeh, A.M.; Jahanian-Najafabadi, A.; Amiri, F.; Roudkenar, M.H. Transplantation of Umbilical Cord-Derived Mesenchymal Stem Cells Overexpressing Lipocalin 2 Ameliorates Ischemia-Induced Injury and Reduces Apoptotic Death in a Rat Acute Myocardial Infarction Model. Stem Cell Rev. Rep. 2020, 16, 968–978. [Google Scholar] [CrossRef]
- He, H.; Zhao, Z.H.; Han, F.S.; Liu, X.H.; Wang, R.; Zeng, Y.J. Overexpression of protein kinase C ε improves retention and survival of transplanted mesenchymal stem cells in rat acute myocardial infarction. Cell Death Dis. 2016, 7, e2056. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Tao, L.; Yang, Z.; Wang, L. Mesenchymal Stem Cells with eNOS Over-Expression Enhance Cardiac Repair in Rats with Myocardial Infarction. Cardiovasc. Drugs Ther. 2017, 31, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Boon, R.A.; Dimmeler, S. MicroRNAs in myocardial infarction. Nat. Rev. Cardiol. 2015, 12, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Yang, B.; Ma, L.N.; Dong, Y.H. MicroRNA-1 effectively induces differentiation of myocardial cells from mouse bone marrow mesenchymal stem cells. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, M.L.; Fang, Z.F.; Hu, X.Q.; Liu, Q.M.; Liu, Z.J.; Tang, L.; Zhao, Y.S.; Zhou, S.H. Overexpression of MicroRNA-1 improves the efficacy of mesenchymal stem cell transplantation after myocardial infarction. Cardiology 2013, 125, 18–30. [Google Scholar] [CrossRef]
- Chen, C.; Chen, T.; Li, Y.; Xu, Y. miR-19a/19b improves the therapeutic potential of mesenchymal stem cells in a mouse model of myocardial infarction. Gene Ther. 2020. [Google Scholar] [CrossRef]
- Tu, Y.; Qiu, Y.; Liu, L.; Huang, T.; Tang, H.; Liu, Y.; Guo, W.; Jiang, H.; Fan, Y.; Yu, B. mi R -15a/15b Cluster Modulates Survival of Mesenchymal Stem Cells to Improve Its Therapeutic Efficacy of Myocardial Infarction. J. Am. Heart Assoc. 2019, 8, e010157. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Zhou, S.H. Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiol. J. 2011, 18, 675–681. [Google Scholar] [CrossRef]
- Huang, F.; Zhu, X.; Hu, X.Q.; Fang, Z.F.; Tang, L.; Lu, X.L.; Zhou, S.H. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int. J. Mol. Med. 2013, 31, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Huang, W.; Feng, Y.; Cai, W.; Wang, Y.; Wang, X.; Liang, J.; Wani, M.; Chen, J.; Zhu, P.; et al. MicroRNA-377 regulates mesenchymal stem cell-induced angiogenesis in ischemic hearts by targeting VEGF. PLoS ONE 2014, 9, e104666. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Lei, M.; Hu, W.; Han, S.; Wang, Q. A brief review: The therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res. Ther. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhang, W.; Nam, Y.J. Stoichiometric optimization of Gata4, Hand2, Mef2c, and Tbx5 expression for contractile cardiomyocyte reprogramming. Sci. Rep. 2019, 9, 14970. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, T.M.; Stone, N.R.; Berry, E.C.; Radzinsky, E.; Huang, Y.; Pratt, K.; Ang, Y.S.; Yu, P.; Wang, H.; Tang, S.; et al. Chemical Enhancement of In Vitro and In Vivo Direct Cardiac Reprogramming. Circulation 2017, 135, 978–995. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Yin, C.; Asfour, H.; Chen, O.; Li, Y.; Bursac, N.; Liu, J.; Qian, L. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ. Res. 2015, 116, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, S.; Miki, K.; Takaki, T.; Okubo, C.; Hatani, T.; Chonabayashi, K.; Nishikawa, M.; Takei, I.; Oishi, A.; Narita, M.; et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016, 6, 19111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachibana, A.; Santoso, M.R.; Mahmoudi, M.; Shukla, P.; Wang, L.; Bennett, M.; Goldstone, A.B.; Wang, M.; Fukushi, M.; Ebert, A.D.; et al. Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circ. Res. 2017, 121, e22–e36. [Google Scholar] [CrossRef] [PubMed]
- Miwa, T.; Idiris, A.; Kumagai, H. A novel cardiac differentiation method of a large number and uniformly-sized spheroids using microfabricated culture vessels. Regen. Ther. 2020, 15, 18–26. [Google Scholar] [CrossRef]
- Yassa, M.E.; Mansour, I.A.; Sewelam, N.I.; Hamza, H.; Gaafar, T. The impact of growth factors on human induced pluripotent stem cells differentiation into cardiomyocytes. Life Sci. 2018, 196, 38–47. [Google Scholar] [CrossRef]
- Später, D.; Hansson, E.M.; Zangi, L.; Chien, K.R. How to make a cardiomyocyte. Development 2014, 141, 4418–4431. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Lian, X.L. Heart regeneration with human pluripotent stem cells: Prospects and challenges. Bioact. Mater. 2020, 5, 74–81. [Google Scholar] [CrossRef]
- Loh, K.M.; Chen, A.; Koh, P.W.; Deng, T.Z.; Sinha, R.; Tsai, J.M.; Barkal, A.A.; Shen, K.Y.; Jain, R.; Morganti, R.M.; et al. Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell 2016, 166, 451–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgkinson, C.P.; Bareja, A.; Gomez, J.A.; Dzau, V.J. Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ. Res. 2016, 118, 95–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halloin, C.; Schwanke, K.; Löbel, W.; Franke, A.; Szepes, M.; Biswanath, S.; Wunderlich, S.; Merkert, S.; Weber, N.; Osten, F.; et al. Continuous WNT Control Enables Advanced hPSC Cardiac Processing and Prognostic Surface Marker Identification in Chemically Defined Suspension Culture. Stem Cell Rep. 2019, 13, 366–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szaraz, P.; Gratch, Y.S.; Iqbal, F.; Librach, C.L. In Vitro Differentiation of Human Mesenchymal Stem Cells into Functional Cardiomyocyte-like Cells. J. Vis. Exp. JoVE 2017, 126, e55757. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, D.; Acosta Torres, Z.; Ng, T.K.; Choy, K.W.; Pang, C.P.; Cheung, H.S. Cardiomyogenesis of periodontal ligament-derived stem cells by dynamic tensile strain. Cell Tissue Res. 2017, 367, 229–241. [Google Scholar] [CrossRef]
- Marino, F.; Scalise, M.; Cianflone, E.; Mancuso, T.; Aquila, I.; Agosti, V.; Torella, M.; Paolino, D.; Mollace, V.; Nadal-Ginard, B.; et al. Role of c-Kit in Myocardial Regeneration and Aging. Front. Endocrinol. 2019, 10, 371. [Google Scholar] [CrossRef]
- Vicinanza, C.; Aquila, I.; Cianflone, E.; Scalise, M.; Marino, F.; Mancuso, T.; Fumagalli, F.; Giovannone, E.D.; Cristiano, F.; Iaccino, E.; et al. Kit(cre) knock-in mice fail to fate-map cardiac stem cells. Nature 2018, 555, E1–E5. [Google Scholar] [CrossRef]
- Maliken, B.D.; Kanisicak, O.; Karch, J.; Khalil, H.; Fu, X.; Boyer, J.G.; Prasad, V.; Zheng, Y.; Molkentin, J.D. Gata4-Dependent Differentiation of c-Kit(+)-Derived Endothelial Cells Underlies Artefactual Cardiomyocyte Regeneration in the Heart. Circulation 2018, 138, 1012–1024. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.Q.; Wang, L.P.; Yao, Y.H.; Ma, C.Y.; Ding, J.F.; Ye, J.; Meng, X.M.; Li, J.J.; Xu, R.X. Overexpression of Cardiac-Specific Kinase TNNI3K Promotes Mouse Embryonic Stem Cells Differentiation into Cardiomyocytes. Cell. Physiol. Biochem. 2017, 41, 381–398. [Google Scholar] [CrossRef]
- Shen, X.; Pan, B.; Zhou, H.; Liu, L.; Lv, T.; Zhu, J.; Huang, X.; Tian, J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J. Biomed. Sci. 2017, 24, 29. [Google Scholar] [CrossRef]
- Zhao, M.; Fan, C.; Ernst, P.J.; Tang, Y.; Zhu, H.; Mattapally, S.; Oduk, Y.; Borovjagin, A.V.; Zhou, L.; Zhang, J.; et al. Y-27632 preconditioning enhances transplantation of human-induced pluripotent stem cell-derived cardiomyocytes in myocardial infarction mice. Cardiovasc. Res. 2019, 115, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liang, J.; Huang, W.; Guo, L.; Cai, W.; Wang, L.; Paul, C.; Yang, H.T.; Kim, H.W.; Wang, Y. Electrical Stimulation Enhances Cardiac Differentiation of Human Induced Pluripotent Stem Cells for Myocardial Infarction Therapy. Antioxid. Redox Signal. 2018, 28, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Fei, Q.; Xiao, H.; Wang, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X.; Wang, K.; Wang, N. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J. Cell. Physiol. 2019, 234, 17690–17703. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, F.; Feyen, D.A.; van den Hoogen, P.; van Laake, L.W.; van Eeuwijk, E.C.; Hoefer, I.; Pasterkamp, G.; Chamuleau, S.A.; Grundeman, P.F.; Doevendans, P.A.; et al. Intramyocardial stem cell injection: Go(ne) with the flow. Eur. Heart J. 2017, 38, 184–186. [Google Scholar] [CrossRef] [Green Version]
- Templin, C.; Zweigerdt, R.; Schwanke, K.; Olmer, R.; Ghadri, J.R.; Emmert, M.Y.; Müller, E.; Küest, S.M.; Cohrs, S.; Schibli, R.; et al. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: Assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation 2012, 126, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Wu, Q.; Ni, C.; Zhang, P.; Zhong, Z.; Wu, Y.; Wang, Y.; Xu, Y.; Kong, M.; Cheng, H.; et al. Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates. Circ. Res. 2018, 122, 958–969. [Google Scholar] [CrossRef]
- Romagnuolo, R.; Masoudpour, H.; Porta-Sánchez, A.; Qiang, B.; Barry, J.; Laskary, A.; Qi, X.; Massé, S.; Magtibay, K.; Kawajiri, H.; et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Rep. 2019, 12, 967–981. [Google Scholar] [CrossRef] [Green Version]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef]
- Samak, M.; Hinkel, R. Stem Cells in Cardiovascular Medicine: Historical Overview and Future Prospects. Cells 2019, 8, 1530. [Google Scholar] [CrossRef] [Green Version]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raziyeva, K.; Smagulova, A.; Kim, Y.; Smagul, S.; Nurkesh, A.; Saparov, A. Preconditioned and Genetically Modified Stem Cells for Myocardial Infarction Treatment. Int. J. Mol. Sci. 2020, 21, 7301. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197301
Raziyeva K, Smagulova A, Kim Y, Smagul S, Nurkesh A, Saparov A. Preconditioned and Genetically Modified Stem Cells for Myocardial Infarction Treatment. International Journal of Molecular Sciences. 2020; 21(19):7301. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197301
Chicago/Turabian StyleRaziyeva, Kamila, Aiganym Smagulova, Yevgeniy Kim, Saltanat Smagul, Ayan Nurkesh, and Arman Saparov. 2020. "Preconditioned and Genetically Modified Stem Cells for Myocardial Infarction Treatment" International Journal of Molecular Sciences 21, no. 19: 7301. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197301