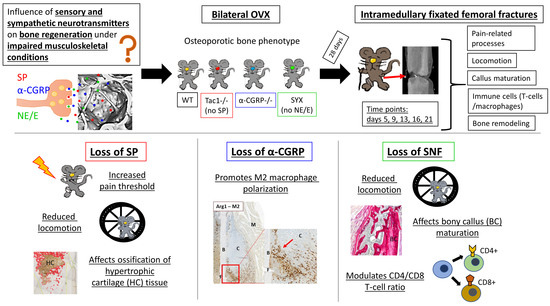
Impact of the Sensory and Sympathetic Nervous System on Fracture Healing in Ovariectomized Mice
Abstract
:1. Introduction
2. Results
2.1. Locomotion Analysis before and after Fracture
2.2. Mechanical Hyperalgesia before and after Fracture
2.3. Maturation of Mesenchymal, Cartilaginous and Bony Callus Tissue
2.4. Hypertrophic Callus Area and Number of TRAP-Positive Callus Cells
2.5. Number of CD4- and CD8-Positive T Cells during Callus Maturation
2.6. Macrophage Subtype Profile Five Days after Fracture
2.7. Serum Analysis of Bone Resorption and Bone Formation Markers
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Operation Procedures
4.3. Locomotion Analysis
4.4. Dynamic Plantar Aesthesiometer Test
4.5. Sample Preparation for Histological Stainings
4.6. Alcian Blue—Sirius Red Staining
4.7. Collagen X (Col X) Staining for Detection of Hypertrophic Chondrocytes
4.8. Staining of Tartrate Resistant Acid Phosphatase (TRAP)-Positive Cells
4.9. Immunohistological Staining for CD4, CD8, F4/80, iNos and Arg1
4.10. Serum Analysis
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lerner, U.H. Neuropeptidergic regulation of bone resorption and bone formation. J. Musculoskelet. Neuronal Interact. 2002, 2, 440–447. [Google Scholar] [PubMed]
- Grassel, S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014, 16, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedermair, T.; Kuhn, V.; Doranehgard, F.; Stange, R.; Wieskotter, B.; Beckmann, J.; Salmen, P.; Springorum, H.R.; Straub, R.H.; Zimmer, A.; et al. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol. 2014, 38, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Muschter, D.; Fleischhauer, L.; Taheri, S.; Schilling, A.F.; Clausen-Schaumann, H.; Grässel, S. Sensory neuropeptides are required for bone and cartilage homeostasis in a murine destabilization-induced osteoarthritis model. Bone 2019. accepted. [Google Scholar] [CrossRef]
- Harrison, S.; Geppetti, P. Substance p. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Severini, C.; Improta, G.; Falconieri-Erspamer, G.; Salvadori, S.; Erspamer, V. The tachykinin peptide family. Pharmacol. Rev. 2002, 54, 285–322. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, M.; Konttinen, Y.T.; Santavirta, S.; Nordsletten, L.; Madsen, J.E.; Almaas, R.; Oestreicher, A.B.; Rootwelt, T.; Polak, J.M. Effect of sciatic nerve section on neural ingrowth into the rat tibial fracture callus. Clin. Orthop. Relat. Res. 1995, 311, 247–257. [Google Scholar]
- Ding, W.G.; Zhang, Z.M.; Zhang, Y.H.; Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Changes of substance P during fracture healing in ovariectomized mice. Regul. Pept. 2010, 159, 28–34. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Shi, X.; Zhao, R.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-kappaB activation, osteoclastogenesis and bone resorption. Bone 2010, 46, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Mrak, E.; Guidobono, F.; Moro, G.; Fraschini, G.; Rubinacci, A.; Villa, I. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by beta-catenin stabilization. J. Cell. Physiol. 2010, 225, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Sample, S.J.; Hao, Z.; Wilson, A.P.; Muir, P. Role of calcitonin gene-related peptide in bone repair after cyclic fatigue loading. PLoS ONE 2011, 6, e20386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schinke, T.; Liese, S.; Priemel, M.; Haberland, M.; Schilling, A.F.; Catala-Lehnen, P.; Blicharski, D.; Rueger, J.M.; Gagel, R.F.; Emeson, R.B.; et al. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J. Bone Miner. Res. 2004, 19, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 2018, 98, 1083–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.H.; Brennan, T.C.; Muir, M.M.; Mason, R.S. Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. J. Cell. Physiol. 2009, 220, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.J.; Landao-Bassonga, E.; Ralston, S.H.; Idris, A.I. Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch. Biochem. Biophys. 2009, 482, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Opolka, A.; Straub, R.H.; Pasoldt, A.; Grifka, J.; Grassel, S. Substance P and norepinephrine modulate murine chondrocyte proliferation and apoptosis. Arthritis Rheum. 2012, 64, 729–739. [Google Scholar] [CrossRef]
- Motyl, K.J.; Beauchemin, M.; Barlow, D.; Le, P.T.; Nagano, K.; Treyball, A.; Contractor, A.; Baron, R.; Rosen, C.J.; Houseknecht, K.L. A novel role for dopamine signaling in the pathogenesis of bone loss from the atypical antipsychotic drug risperidone in female mice. Bone 2017, 103, 168–176. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I.; Bajayo, A.; Kreisel, T.; Feldman, S.; Tam, J.; Trembovler, V.; Csernus, V.; Shohami, E.; Bab, I. Depression induces bone loss through stimulation of the sympathetic nervous system. Proc. Natl. Acad. Sci. USA 2006, 103, 16876–16881. [Google Scholar] [CrossRef] [Green Version]
- Sachs, C.; Jonsson, G. Mechanisms of action of 6-hydroxydopamine. Biochem. Pharmacol. 1975, 24, 1–8. [Google Scholar] [CrossRef]
- Harle, P.; Mobius, D.; Carr, D.J.; Scholmerich, J.; Straub, R.H. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum. 2005, 52, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Haffner-Luntzer, M.; Hankenson, K.D.; Ignatius, A.; Pfeifer, R.; Khader, B.A.; Hildebrand, F.; van Griensven, M.; Pape, H.-C.; Lehmicke, M. Review of animal models of comorbidities in fracture-healing research. J. Orthop. Res. 2019, 37, 2491–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riggs, B.L.; Khosla, S.; Melton, L.J., 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L. Endocrine causes of age-related bone loss and osteoporosis. Novartis Found. Symp. 2002, 242, 247–259, discussion 260–244. [Google Scholar] [PubMed]
- Diwan, A.D.; Wang, M.X.; Jang, D.; Zhu, W.; Murrell, G.A.C. Nitric oxide modulates fracture healing. J. Bone Miner. Res. 2000, 15, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, C.; Meliconi, R.; Frizziero, L.; Silvestri, T.; Pulsatelli, L.; Mazzetti, I.; Borzì, R.M.; Uguccioni, M.; Facchini, A. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1998, 41, 2165–2174. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Hak, D.J.; Toker, S.; Yi, C.; Toreson, J. The influence of fracture fixation biomechanics on fracture healing. Orthopedics 2010, 33, 752–755. [Google Scholar] [CrossRef] [Green Version]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Augat, P.; Merk, J.; Ignatius, A.; Margevicius, K.; Bauer, G.; Rosenbaum, D.; Claes, L. Early, full weightbearing with flexible fixation delays fracture healing. Clin. Orthop. Relat. Res. (1976–2007) 1996, 328, 194–202. [Google Scholar] [CrossRef]
- Hankemeier, S.; Grassel, S.; Plenz, G.; Spiegel, H.U.; Bruckner, P.; Probst, A. Alteration of fracture stability influences chondrogenesis, osteogenesis and immigration of macrophages. J. Orthop. Res. 2001, 19, 531–538. [Google Scholar] [CrossRef]
- Li, W.W.; Guo, T.Z.; Liang, D.Y.; Sun, Y.; Kingery, W.S.; Clark, J.D. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology 2012, 116, 882–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt-Pichat, B.; Lafage-Proust, M.H.; Duboeuf, F.; Laroche, N.; Itzstein, C.; Vico, L.; Delmas, P.D.; Chenu, C. Dramatic decrease of innervation density in bone after ovariectomy. Endocrinology 2005, 146, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Gaszner, B. Role of neuropeptides in anxiety, stress, and depression: From animals to humans. Neuropeptides 2013, 47, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.J.; Ashina, M. Calcitonin gene-related peptide and pain: A systematic review. J. Headache Pain 2017, 18, 34. [Google Scholar] [CrossRef] [Green Version]
- John, N.W.; Judith, A.S.; Jun-Ming, Z.; Hans-Georg, S. The Sympathetic Nervous System and Pain; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Jänig, W.; Baron, R. Sympathetic nervous system and pain. In Encyclopedia of Pain; Schmidt, R.F., Willis, W.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 2356–2366. [Google Scholar]
- Derevenco, P.; Stoica, N.; Sovrea, I.; Imreh, S. Central and peripheral effects of 6-hydroxydopamine on exercise performance in rats. Psychoneuroendocrinology 1986, 11, 141–153. [Google Scholar] [CrossRef]
- Niedermair, T.; Schirner, S.; Seebröker, R.; Straub, R.H.; Grässel, S. Substance P modulates bone remodeling properties of murine osteoblasts and osteoclasts. Sci. Rep. 2018, 8, 9199. [Google Scholar] [CrossRef]
- Komnenou, A.; Karayannopoulou, M.; Polizopoulou, Z.S.; Constantinidis, T.C.; Dessiris, A. Correlation of serum alkaline phosphatase activity with the healing process of long bone fractures in dogs. Vet. Clin. Pathol. 2005, 34, 35–38. [Google Scholar] [CrossRef]
- Nyman, M.T.; Paavolainen, P.; Forsius, S.; Lamberg-Allardt, C. Clinical evaluation of fracture healing by serum osteocalcin and alkaline phosphatase. Ann. Chir. Gynaecol. 1991, 80, 289–293. [Google Scholar]
- Schmidt-Bleek, K.; Schell, H.; Lienau, J.; Schulz, N.; Hoff, P.; Pfaff, M.; Schmidt, G.; Martin, C.; Perka, C.; Buttgereit, F.; et al. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 2014, 8, 120–130. [Google Scholar] [CrossRef]
- Könnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.-D.; Radbruch, A.; Duda, G.N.; et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014, 64, 155–165. [Google Scholar] [CrossRef]
- Di Rosa, F.; Pabst, R. The bone marrow: A nest for migratory memory T cells. Trends Immunol. 2005, 26, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Marceau, A.-A.; Flamand, L.; Poubelle, P.E. Normal human primary CD4+ T lymphocytes synthesize and release functional osteoprotegerin in vitro. Lab. Investig. 2007, 88, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raggatt, L.J.; Wullschleger, M.E.; Alexander, K.A.; Wu, A.C.K.; Millard, S.M.; Kaur, S.; Maugham, M.L.; Gregory, L.S.; Steck, R.; Pettit, A.R. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am. J. Pathol. 2014, 184, 3192–3204. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Murphy, K.M.; Sher, A. Functional diversity of helper T lymphocytes. Nature 1996, 383, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Abdelmagid, S.M.; Barbe, M.F.; Safadi, F.F. Role of inflammation in the aging bones. Life Sci. 2015, 123, 25–34. [Google Scholar] [CrossRef]
- Schlundt, C.; Schell, H.; Goodman, S.B.; Vunjak-Novakovic, G.; Duda, G.N.; Schmidt-Bleek, K. Immune modulation as a therapeutic strategy in bone regeneration. J. Exp. Orthop. 2015, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Wang, G.; Qiao, L.; Ge, Q.; Chen, D.; Xu, Z.; Shi, D.; Dai, J.; Qin, J.; Teng, H.; et al. Age-dependent variations of cancellous bone in response to ovariectomy in C57BL/6J mice. Exp. Ther. Med. 2018, 15, 3623–3632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouxsein, M.L.; Myers, K.S.; Shultz, K.L.; Donahue, L.R.; Rosen, C.J.; Beamer, W.G. Ovariectomy-induced bone loss varies among inbred strains of mice. J. Bone Miner. Res. 2005, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Kalu, D.N.; Chen, C. Ovariectomized murine model of postmenopausal calcium malabsorption. J. Bone Miner. Res. 1999, 14, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Klinck, J.; Boyd, S.K. The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif. Tissue Int. 2008, 83, 70–79. [Google Scholar] [CrossRef]
- Zimmer, A.; Zimmer, A.M.; Baffi, J.; Usdin, T.; Reynolds, K.; Konig, M.; Palkovits, M.; Mezey, E. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc. Natl. Acad. Sci. USA 1998, 95, 2630–2635. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.T.; Son, Y.J.; Lee, J.; Jetton, T.L.; Shiota, M.; Moscoso, L.; Niswender, K.D.; Loewy, A.D.; Magnuson, M.A.; Sanes, J.R.; et al. Mice lacking alpha-calcitonin gene-related peptide exhibit normal cardiovascular regulation and neuromuscular development. Mol. Cell. Neurosci. 1999, 14, 99–120. [Google Scholar] [CrossRef]
- Sophocleous, A.; Idris, A.I. Rodent models of osteoporosis. Bonekey Rep. 2014, 3, 614. [Google Scholar] [CrossRef] [Green Version]
- Holstein, J.H.; Menger, M.D.; Culemann, U.; Meier, C.; Pohlemann, T. Development of alocking femur nail for mice. J. Biomech. 2007, 40, 215–219. [Google Scholar] [CrossRef]
- Bertrand, J.; Stange, R.; Hidding, H.; Echtermeyer, F.; Nalesso, G.; Godmann, L.; Timmen, M.; Bruckner, P.; Dell’Accio, F.; Raschke, M.J.; et al. Syndecan 4 supportsbone fracture repair, but not fetal skeletal development, in mice. Arthritis Rheum. 2013, 65, 743–752. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharm. Pharm. 2010, 1, 87–93. [Google Scholar] [CrossRef] [Green Version]







| Serum Marker | CTX I (µg/mL) | ALP (IU/L = µmol/(L*min)) | ||
|---|---|---|---|---|
| Time Point | Day 9 | Day 16 | Day 9 | Day 16 |
| Sample | ||||
| WT | 5.035 ± 1.342 | 6.377 ± 1.208 | 269.5 ± 22.56 | 352.4 ± 47.82 |
| Tac1−/− | 6.707 ± 2.82 | 5.088 ± 1.257 | 275.5 ± 25.15 | 348.8 ± 64.69 |
| α-CGRP−/− | 8.238 ± 3.47 | 6.507 ± 0.497 | 244.6 ± 27.02 | 342.4 ± 73.57 |
| SYX | 5.638 ± 1.382 | 6.665 ± 1.841 | 291.6 ± 27.88 | 406.5 ± 36.28 + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedermair, T.; Straub, R.H.; Brochhausen, C.; Grässel, S. Impact of the Sensory and Sympathetic Nervous System on Fracture Healing in Ovariectomized Mice. Int. J. Mol. Sci. 2020, 21, 405. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020405
Niedermair T, Straub RH, Brochhausen C, Grässel S. Impact of the Sensory and Sympathetic Nervous System on Fracture Healing in Ovariectomized Mice. International Journal of Molecular Sciences. 2020; 21(2):405. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020405
Chicago/Turabian StyleNiedermair, Tanja, Rainer H. Straub, Christoph Brochhausen, and Susanne Grässel. 2020. "Impact of the Sensory and Sympathetic Nervous System on Fracture Healing in Ovariectomized Mice" International Journal of Molecular Sciences 21, no. 2: 405. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020405