Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.2.1. Inclusion Criteria:
2.2.2. Exclusion Criteria:
2.3. Data Extraction and Quality Assessment
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Different Animal Models
3.2.2. Behavioral Test Analysis
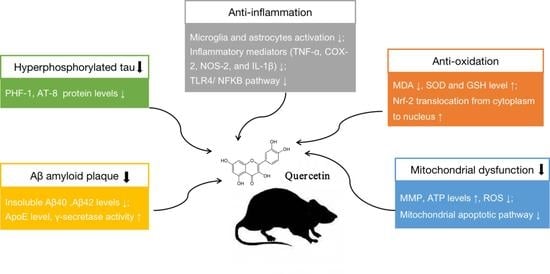
3.2.3. Neuroprotective Mechanisms Analysis
3.3. Methodological Quality Assessment
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Ohta, K. Quercetin Regulates the Integrated Stress Response to Improve Memory. Int. J. Mol. Sci. 2019, 20, 2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Zhu, Q.; Zhang, S.; OuYang, D.; Lu, J.H. Resveratrol in experimental Alzheimer’s disease models: A systematic review of preclinical studies. Pharmacol. Res. 2019, 150, 104476. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.S.; Singh, V.P.; Satyanarayan, P.S.; Jain, N.K.; Singh, A.; Kulkarni, S.K. Protective effect of flavonoids against aging-and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology 2003, 69, 59–67. [Google Scholar] [CrossRef]
- Wang, D.M.; Li, S.Q.; Wu, W.L.; Zhu, X.Y.; Wang, Y.; Yuan, H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef]
- Hayakawa, M.; Itoh, M.; Ohta, K.; Li, S.; Ueda, M.; Wang, M.X.; Nishida, E.; Islam, S.; Suzuki, C.; Ohzawa, K.; et al. Quercetin reduces eIF2α phosphorylation by GADD34 induction. Neurobiol. Aging 2015, 36, 2509–2518. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Munoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hu, J.; Zhong, L.; Wang, N.; Yang, L.; Liu, C.C.; Li, H.; Wang, X.; Zhou, Y.; Zhang, Y.; et al. Quercetin stabilizes apolipoprotein E and reduces brain Aβ levels in amyloid model mice. Neuropharmacology 2016, 108, 179–192. [Google Scholar] [CrossRef]
- Sun, D.; Li, N.; Zhang, W.; Zhao, Z.; Mou, Z.; Huang, D.; Liu, J.; Wang, W. Design of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s disease. Colloids Surf. B Biointerfaces 2016, 148, 116–129. [Google Scholar] [CrossRef]
- Puerta, E.; Suárez-Santiago, J.E.; Santos-Magalhães, N.S.; Ramirez, M.J.; Irache, J.M. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int. J. Pharm. 2017, 517, 50–57. [Google Scholar]
- Vargas-Restrepo, F.; Sabogal-Guáqueta, A.M.; Cardona-Gómez, G.P. Quercetin ameliorates inflammation in CA1 hippocampal region in aged triple transgenic Alzheimer’ s disease mice model. Biomédica 2018, 38, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O. Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Rishitha, N.; Muthuraman, A. Therapeutic evaluation of solid lipid nanoparticle of quercetin in pentylenetetrazole induced cognitive impairment of zebrafish. Life Sci. 2018, 199, 80–87. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Q.; Yu, Q. Quercetin enrich diet during the early-middle not middle-late stage of alzheimer’s disease ameliorates cognitive dysfunction. Am. J. Transl. Res. 2018, 10, 1237. [Google Scholar]
- Karimipour, M.; Rahbarghazi, R.; Tayefi, H.; Shimia, M.; Ghanadian, M.; Mahmoudi, J.; Bagheri, H.S. Quercetin promotes learning and memory performance concomitantly with neural stem/progenitor cell proliferation and neurogenesis in the adult rat dentate gyrus. Int. J. Dev. Neurosci. 2019, 74, 18–26. [Google Scholar] [CrossRef]
- Paula, P.C.; Angelica Maria, S.G.; Luis, C.H.; Gloria Patricia, C.G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tian, Q.; Li, Z.; Dang, M.; Lin, Y.; Hou, X. Activation of Nrf2 signaling by sitagliptin and quercetin combination against β-amyloid induced Alzheimer’s disease in rats. Drug Dev. Res. 2019, 80, 837–845. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. Bmc Med Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Habtemariam, S. Rutin as a natural therapy for Alzheimer’s disease: Insights into its mechanisms of action. Curr. Med. Chem. 2016, 23, 860–873. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhuang, X.; Lu, J. Neuroprotective effects of baicalein in animal models of Parkinson’s disease: A systematic review of experimental studies. Phytomedicine 2019, 55, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.A.; Gama Sosa, M.A.; De Gasperi, R. Mouse models of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1171–1183. [Google Scholar]
- Men, K.; Duan, X.; Wei, X.W.; Gou, M.L.; Huang, M.J.; Chen, L.J.; Qian, Z.Y.; Wei, Y.Q. Nanoparticle-delivered quercetin for cancer therapy. Anti-Cancer Agents Med. Chem. 2014, 14, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019. [Google Scholar] [CrossRef] [PubMed]





| Author (Year) | Animal Data | Quercetin Administration | Outcome Measure | Pharmacological Activities (Mechanisms) |
|---|---|---|---|---|
| Patil CS (2003) [5] | LPS-induced mice AD model (Swiss mice, male&female, 3 months old, 15–20 g and 16 months old, 35–40 g) | Dosage: 25, 50, and 100 mg/kg/day; Ad: i.p.; Duration: 7 days | Behavioral test (elevated plus maze, locomotor activity test, passive avoidance task, Rota-Rod test) | Prevented the cognitive impairment (oxidative stress↓) |
| Wang DM (2014) [6] | APPswe/PS1 dE9 transgenic AD mice (C57/BL) (male&female, 3 months old) | Dosage: 20 and 40 mg/kg/day; Ad: p.o.; Duration:16 weeks | Behavioral test (Novel Object Recognition Test, Morris Water Maze); Thioflavine S staining (Aβ deposition); WB (AMPK and p-AMPK levels) | Lessened cognitive deficits, reduced Aβ plaques and ameliorated mitochondrial dysfunction (AMPK activity↑) |
| Hayakawa M (2015) [7] | APP23 AD mice model (8 weeks old) | Dosage: 20 mg/day; Ad: p.o.; Duration: 4 weeks | Behavioral test (Contextual and auditory fear conditioning test); WB and ELISA (Aβ1-42, GADD34, ATF4, eIF2 a, etc.) | Improved memory (p-eIF2 a↓ and ATF4↓ through GADD34 induction) |
| Sabogal-Guáqueta AM (2015) [8] | Homozygous 3 xTg-AD mice (male&female, 18-21 months old) | Dosage: 25 mg/kg/2 days; Ad: i.p.; Duration: 3 months | Behavioral test (Elevated plus maze, Morris Water Maze); Immunohistochemistry (NeuN, βA, AT8, GFAP and Iba-1); WB (AT8, tau 5 and PHF-1 levels); ELISA (CTFα, CTFβ and βA1-40,42) | Reversed histological hallmarks of AD and protected cognitive and emotional function |
| Zhang X (2016) [9] | 5XFAD transgenic mice (male&female, 6–8 weeks old) | Dosage: 500 mg/kg/day; Ad: p.o.; Duration: 10 days | Immunohistochemistry for Aβ; WB and qRT-PCR for apoE; ELISA (Aβ40 and Aβ42) | Increased brain apoE and reduced insoluble Aβ levels (inhibited apoE degradation) |
| Sun D (2016) [10] | APP/PS1 transgenic AD mice | Dosage: 10, 20 and 30 mg/kg (PLGA@QT NPs); Ad: i.v.; Duration: 30 days | Behavioral test (Morris Water Maze, Novel Object Recognition Test) | PLGA-functionalized quercetin (PLGA@QT) NPs ameliorated cognition and memory impairments |
| Moreno LCGEI (2017) [11] | SAMP1&SAMP8 mice (Male, 5 months old, 28–30 g) | Dosage: 25 mg/kg/day (Q) and 25 mg/kg/2 days (NPQ); Ad: p.o.; Duration: 2 months | Behavioral test (Morris Water Maze, Open field test, Rotarod test, Marble burying test); WB (GFAP, CD11) | Nanoencapsulaed quercetin (NPQ) improved the cognition and memory impairments (GFAP↓) |
| Vargas-Restrepo F (2018) [12] | Homozygous 3xTg-AD mice (male&female, 18–21 months old) | Dosage: 25 mg/Kg/48 h; Ad: i.p.; Duration: 3 months | Immunofluorescence (Iba-1 and βA); immunohistochemistry (GFAP, iNOS and COX-2) | Anti-inflammatory effect in CA1 hippocampal region |
| Khan A (2018) [13] | LPS-induced mice AD model (male, 8 weeks old, 25–30 g) | Dosage: 30 mg/kg/day; Ad: i.p.; Duration: 2 weeks | Behavioral test (Morris Water Maze, Y-maze); WB (GFAP, Iba-1, TLR4/NFKB, TNF-α, Caspase-3, etc.); Immunofluorescence (GFAP, Iba-1, p-NFKB, IL-1β, Caspase-3, etc.); Nissl staining | Reduced gliosis, prevented neuroinflammation in cortex and hippocampus, rescued the mitochondrial apoptotic pathway and neuronal degeneration (cyto. C↓, caspase-3↓ and PARP-1↓) |
| Rishitha N (2018) [14] | PTZ-induced Zebrafish AD model (adult male, <8 months old, 1.0–1.2 g) | Dosage: 5 and 10 mg/kg (Q and SLN-Q); Ad: i.p.; Duration: single | Light and dark chamber test; Partition preference test; Three horizontal compartment test; Spectroscopic method (GSH, TBARS, AChE levels) | Solid lipid nanoparticle of quercetin (SLN-Q) attenuated neurocognitive impairments along with amelioration of oxidative biomarker changes |
| Lu Y (2018) [15] | APP/PS1 transgenic AD mice (13 months old) | Dosage: 2 mg/g diet; Ad: p.o.; Duration: 9 or 13 months | Behavioral test (Morris Water Maze); Immunostaining (GFAP, 6E10); WB (APP, CTFβ, GFAP, etc); RT-qPCR (BACE1, PS1, Hevin, SPARC, Smad2, STAT3) | Ameliorated cognitive dysfunction only during early-middle stage of AD (astrogliosis↓, Aβ↓) |
| Karimipour M (2019) [16] | Aβ-injection rats AD model (Adult male Wistar rats, 350–400 g) | Dosage: 40 mg/kg/day; Ad: p.o.; Duration: 1 month | Morris water maze behavioral test; Immunohistochemistry (BrdU, DCX); Immunostaining (BrdU/NeuN double positive cells); RT-qPCR (BDN, NGF, CREB and ERG-1) | Increased proliferating neural stem/progenitor cells, enhanced adult neurogenesis (BDNF, NGF, CREB and EGR-1 genes expression↑) |
| Paula PC (2019) [17] | Homozygous 3xTg-AD mice (male&female, 6 months old) | Dosage: 100 mg/kg/48 h; Ad: p.o.; Duration: 12 months | Behavioral test (Elevated plus maze, Morris Water Maze); Immunohistochemistry (Aβ, AT-8) | Reduced β-amyloidosis, decreased tauopathy in hippocampus and amygdala, affected cognitive recovery |
| Li Y (2019) [18] | Aβ-injection rats AD model (male Sprague– Dawley rats, 220–280 g) | Dosage: 100 mg/kg/day; Ad: p.o.; Duration: 18 days | Morris water maze behavioral test; Estimation of oxidative stress (MDA level, SOD, CAT and GSH activity); Immunohistochemistry for Aβ; WB (Nrf2 and HO-1) | Promoted reversal of neuronal damage, Improved cognitive memory (Aβ1-42↓, antioxidant activity and Nrf2/HO-1 pathway↑) |
| Model | Mechanism | Main Uses of the Model | Disadvantage |
|---|---|---|---|
| Aβ-induced | Neurotoxicity of Aβ species | Studying Aβ peptide aggregation and deposition, and its acute toxic effect in AD | Not reproducing the progressive neurodegeneration process as an acute model |
| LPS-induced | Inducing proinflammatory mediators, activating astrocytes and microglia | Simulating neuroinflammation and synaptic/memory dysfunction of AD | Lack of Aβ plaque accumulation and NFT formation |
| PTZ-induced | Activating free radicals and apoptotosis, modulating neurotransmitters metabolisms | Simulating oxidative damage, motor impairment as well as memory dysfunction of AD | Not replicating the histological hallmarks of AD |
| Senescence acceleratedmouse | Naturally rapid aging mouse model | Studying the mechanism of age-related spatial learning and memory deficits | Short lifecycle not supporting long-term animal experiments |
| APP/PS1/tau- transgenic | Aβ accumulation, NFT formation in the brain | Studying the role of APP and tau protein in the development of AD | Lack of APP and tau metabolism changes |
| Study | A | B | C | D | E | F | G | H | I | J | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patil CS (2003) [5] | NC | Y | NC | Y | NC | NC | NC | Y | Y | NC | 4 |
| Wang DM (2014) [6] | Y | Y | NC | NC | NC | Y | NC | Y | Y | Y | 6 |
| Hayakawa M (2015) [7] | NC | NC | NC | Y | NC | NC | NC | N | Y | Y | 3 |
| Sabogal- Guáqueta AM (2015) [8] | NC | Y | NC | Y | NC | NC | NC | N | Y | Y | 4 |
| Zhang X (2016) [9] | NC | Y | NC | Y | NC | NC | NC | N | Y | NC | 3 |
| Sun D (2016) [10] | NC | NC | NC | Y | NC | NC | NC | NC | Y | NC | 2 |
| Moreno LCGEI (2017) [11] | NC | Y | NC | Y | N | NC | NC | NC | Y | Y | 4 |
| Vargas-Restrepo F (2018) [12] | Y | Y | NC | Y | NC | Y | NC | NC | Y | Y | 6 |
| Khan A (2018) [13] | NC | N | NC | Y | N | NC | NC | Y | Y | Y | 4 |
| Rishitha N (2018) [14] | NC | NC | NC | Y | NC | Y | NC | Y | Y | NC | 4 |
| Lu Y (2018) [15] | NC | NC | NC | Y | NC | NC | NC | Y | Y | NC | 3 |
| Karimipur M (2019) [16] | Y | Y | NC | Y | NC | NC | NC | Y | Y | Y | 6 |
| Paula PC (2019) [17] | NC | Y | NC | Y | NC | NC | NC | N | Y | Y | 4 |
| Li Y (2019) [18] | NC | NC | NC | Y | NC | Y | NC | Y | Y | Y | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-W.; Chen, J.-Y.; Ouyang, D.; Lu, J.-H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 493. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020493
Zhang X-W, Chen J-Y, Ouyang D, Lu J-H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. International Journal of Molecular Sciences. 2020; 21(2):493. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020493
Chicago/Turabian StyleZhang, Xiao-Wen, Jia-Yue Chen, Defang Ouyang, and Jia-Hong Lu. 2020. "Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies" International Journal of Molecular Sciences 21, no. 2: 493. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020493