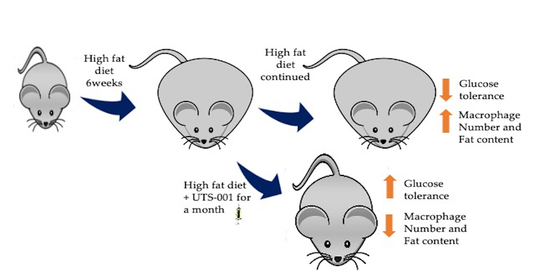
Impact of A Cargo-Less Liposomal Formulation on Dietary Obesity-Related Metabolic Disorders in Mice
Abstract
:1. Introduction
2. Results
2.1. In Vivo Studies
2.1.1. Anthropometric and Metabolic Parameters
2.1.2. Inflammatory Markers
2.1.3. mRNA Expression of Metabolic Markers in Livertitle
2.1.4. mRNA Expression of Metabolic Markers in the Fat
2.1.5. mRNA Expression of Thermogenesis Markers in Brown Fat
2.2. Effect of UTS-001 on Uptake of 2-(N-(7-Nitrobenz-2-oxa-1, 3-Diazol-4-yl) Amino)-2 Deoxyglucose (2-NBDG), Number and Diameter of Adipocytes In Vitro
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.1.1. Bioassays
4.1.2. Immunohistochemistry
4.1.3. Quantitative Real-Time PCR
4.2. Cell Culture Studies and Cellular 2-NBDG Uptake
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IL | Interleukin |
| FASN | Fatty acid synthase |
| SREBP-1c | Sterol regulatory element-binding protein 1 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| FOXO-1 | Forkhead box protein O1 |
| ATGL | Adipose triglyceride lipase |
| TNF-α | Tumor necrosis factor-alpha |
| UCP-1 | Uncoupling protein-1 |
| UCP-3 | Uncoupling protein-3 |
| PPARγ | Peroxisome Proliferator Activated Receptor Gamma |
| TLR4 | Toll-like receptor-4 |
| CPT1a | Carnitine palmitoyltransferase 1a |
| PGC1 α | Peroxisome proliferator-activated receptor gamma coactivator 1-α |
| GLUT 2 | Glucose transporter 2 |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Xie, B.; Waters, M.J.; Schirra, H.J. Investigating potential mechanisms of obesity by metabolomics. J. Biomed. Biotechnol. 2012, 2012, 805683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anari, R.; Amani, R.; Latifi, S.M.; Veissi, M.; Shahbazian, H. Association of obesity with hypertension and dyslipidemia in type 2 diabetes mellitus subjects. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Lagerros, Y.T.; Rössner, S. Obesity management: What brings success? Adv. Gastroenterol. 2013, 6, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemstra, M.; Bird, Y.; Nwankwo, C.; Rogers, M.; Moraros, J. Weight loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Prefer. Adherence 2016, 10, 1547–1559. [Google Scholar]
- Hainer, V. Comparative Efficiency and Safety of Pharmacological Approaches to the Management of Obesity. Diabetes Care 2011, 34, S349. [Google Scholar] [CrossRef] [Green Version]
- Misra, M. Obesity pharmacotherapy: Current perspectives and future directions. Curr. Cardiol. Rev. 2013, 9, 33–54. [Google Scholar]
- Sibuyi, N.R.S.; Moabelo, K.L.; Meyer, M.; Onani, M.O.; Dube, A.; Madiehe, A.M. Nanotechnology advances towards development of targeted-treatment for obesity. J. Nanobiotechnol. 2019, 17, 122. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: A systematic review. BMC Med. 2016, 14, 191. [Google Scholar] [CrossRef] [Green Version]
- Lupoli, R.; Lembo, E.; Saldalamacchia, G.; Avola, C.K.; Angrisani, L.; Capaldo, B. Bariatric surgery and long-term nutritional issues. Worldj. Diabetes 2017, 8, 464–474. [Google Scholar] [CrossRef]
- Coenen, K.R.; Gruen, M.L.; Chait, A.; Hasty, A.H. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes 2007, 56, 564–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraev, T.; Chen, H.; Morris, M.J. Differential effects of restricted versus unlimited high-fat feeding in rats on fat mass, plasma hormones and brain appetite regulators. J. Neuroendocr. 2009, 21, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Isakson, P.; Hammarstedt, A.; Gustafson, B.; Smith, U. Impaired Preadipocyte Differentiation in Human Abdominal Obesity. Diabetes 2009, 58, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Investig. 2006, 116, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Kosteli, A.; Sugaru, E.; Haemmerle, G.; Martin, J.F.; Lei, J.; Zechner, R.; Ferrante, A.W., Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Investig. 2010, 120, 3466–3479. [Google Scholar] [CrossRef] [Green Version]
- Schenk, S.; Saberi, M.; Olefsky, J.M. Insulin sensitivity: Modulation by nutrients and inflammation. J. Clin. Investig. 2008, 118, 2992–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Nam, Y.; Chung, Y.H.; Kim, H.R.; Park, E.S.; Chung, S.J.; Kim, J.H.; Sohn, U.D.; Kim, H.C.; Oh, K.W.; et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014, 118, 7–14. [Google Scholar] [CrossRef]
- Tandy, S.; Chung, R.W.S.; Kamili, A.; Wat, E.; Weir, J.M.; Meikle, P.J.; Cohn, J.S. Hydrogenated phosphatidylcholine supplementation reduces hepatic lipid levels in mice fed a high-fat diet. Atherosclerosis 2010, 213, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, Y.K.; Mamrosh, J.L.; Busby, S.A.; Griffin, P.R.; Pathak, M.C.; Ortlund, E.A.; Moore, D.D. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature 2011, 474, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Navder, K.P.; Lieber, C.S. Dilinoleoylphosphatidylcholine Is Responsible for the Beneficial Effects of Polyenylphosphatidylcholine on Ethanol-Induced Mitochondrial Injury in Rats. Biochem. Biophys. Res. Commun. 2002, 291, 1109–1112. [Google Scholar] [CrossRef]
- Van, H.P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid. Sci. Technol. Ejlst 2014, 116, 1088–1107. [Google Scholar]
- Mehra, H.; Hui, C.; Brian, G.O.; Komalla, V. Liposomes and Uses Thereof. Patent Application 2019903904, 2019. Available online: http://pericles.ipaustralia.gov.au/ols/auspat/applicationDetails.do?applicationNo=2019903904 (accessed on 14 October 2020).
- Fonseca, V.A. Identification and Treatment of Prediabetes to Prevent Progression to Type 2 Diabetes. Clin. Cornerstone 2007, 8, 10–20. [Google Scholar] [CrossRef]
- Chen, H.; Ng, J.P.M.; Tan, Y.; McGrath, K.; Bishop, D.P.; Oliver, B.; Chan, Y.L.; Cortie, M.B.; Milthorpe, B.K.; Valenzuela, S.M. Gold nanoparticles improve metabolic profile of mice fed a high-fat diet. J. Nanobiotechnol. 2018, 16, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef]
- Sharma, B.R.; Rhyu, D.Y. Anti-diabetic effects of Caulerpa lentillifera: Stimulation of insulin secretion in pancreatic β-cells and enhancement of glucose uptake in adipocytes. Asian. Pac. J. Trop. Biomed. 2014, 4, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Weyer, C.; Foley, J.E.; Bogardus, C.; Tataranni, P.A.; Pratley, R.E. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000, 43, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Kühn, C.; Arapogianni, N.E.; Halabalaki, M.; Hempel, J.; Hunger, N.; Wober, J.; Skaltsounis, A.L.; Vollmer, G. Constituents from Cistus salvifolius (Cistaceae) activate peroxisome proliferator-activated receptor-γ but not -δ and stimulate glucose uptake by adipocytes. Planta Med. 2011, 77, 346–353. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, E.J.; Kim, E.D.; Bayaraa, T.; Frost, S.C.; Hyun, C.K. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biol. Pharm. Bull. 2007, 30, 2120–2125. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.R.; Kim, H.J.; Rhyu, D.Y. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J. Transl. Med. 2015, 13, 62. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, K.; Davidsen, J.; Mouritsen, O.G. Biophysical mechanisms of phospholipase A2 activation and their use in liposome-based drug delivery. FEBS Lett. 2002, 531, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Möller, C.; Davis, W.C.; Clark, E.; DeCaprio, A.; Marí, F. Conodipine-P1-3, the First Phospholipases A2 Characterized from Injected Cone Snail Venom. Mol. Cell. Proteom. 2019, 18, 876. [Google Scholar]
- Drzazga, A.; Sowinska, A.; Koziolkiewicz, M. Lysophosphatidylcholine and lysophosphatidylinosiol--novel promissing signaling molecules and their possible therapeutic activity. Acta. Pol. Pharm. 2014, 71, 887–899. [Google Scholar] [PubMed]
- Stanton, M.C.; Chen, S.-C.; Jackson, J.V.; Rojas-Triana, A.; Kinsley, D.; Cui, L.; Fine, J.S.; Greenfeder, S.; Bober, L.A.; Jenh, C.-H. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J. Inflamm. 2011, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayoral Monibas, R.; Johnson, A.M.F.; Osborn, O.; Traves, P.G.; Mahata, S.K. Distinct Hepatic Macrophage Populations in Lean and Obese Mice. Front. Endocrinol. (Lausanne) 2016, 7, 152. [Google Scholar] [CrossRef] [Green Version]
- Cawthorn, W.P.; Sethi, J.K. TNF-alpha and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Taeye, B.M.D.; Novitskaya, T.; McGuinness, O.P.; Gleaves, L.; Medda, M.; Covington, J.W.; Vaughan, D.E. Macrophage TNF-α contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E713–E725. [Google Scholar] [CrossRef]
- Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; Cox, J.; et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N. Eng. Med. 2018, 379, 1529–1539. [Google Scholar]
- Singh, S.; Facciorusso, A.; Singh, A.G.; Vande Casteele, N.; Zarrinpar, A.; Prokop, L.J.; Grunvald, E.L.; Curtis, J.R.; Sandborn, W.J. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195123. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Seol, M.H.; Lee, B.-C. The Effects of Poncirus fructus on Insulin Resistance and the Macrophage-Mediated Inflammatory Response in High Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2019, 20, 2858. [Google Scholar] [CrossRef] [Green Version]
- Cranford, T.L.; Enos, R.T.; Velázquez, K.T.; McClellan, J.L.; Davis, J.M.; Singh, U.P.; Nagarkatti, M.; Nagarkatti, P.S.; Robinson, C.M.; Murphy, E.A. Role of MCP-1 on inflammatory processes and metabolic dysfunction following high-fat feedings in the FVB/N strain. Int. J. Obes. (Lond.) 2016, 40, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Van Ly, D.; Burgess, J.K.; Brock, T.G.; Lee, T.H.; Black, J.L.; Oliver, B.G. Prostaglandins but not leukotrienes alter extracellular matrix protein deposition and cytokine release in primary human airway smooth muscle cells and fibroblasts. Am. J. Physiol Lung. Cell. Mol. Physiol. 2012, 303, L239–L250. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Scambler, T.E.; Smallie, T.; Cunliffe, H.E.; Ross, E.A.; Rosner, D.R.; O’Neil, J.D.; Clark, A.R. Macrophage responses to lipopolysaccharide are modulated by a feedback loop involving prostaglandin E(2), dual specificity phosphatase 1 and tristetraprolin. Sci. Rep. 2017, 7, 4350. [Google Scholar] [CrossRef] [PubMed]
- Emerson, P.; Van Haeften, T.W.; Pimenta, W.; Plummer, E.; Woerle, H.J.; Mitrakou, A.; Szoke, E.; Gerich, J.; Meyer, C. Different pathophysiology of impaired glucose tolerance in first-degree relatives of individuals with type 2 diabetes mellitus. Metab. Clin. Exp. 2009, 58, 602–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O-Sullivan, I.; Zhang, W.; Wasserman, D.H.; Liew, C.W.; Liu, J.; Paik, J.; DePinho, R.A.; Stolz, D.B.; Kahn, C.R.; Schwartz, M.W.; et al. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 2015, 6, 7079. [Google Scholar] [CrossRef] [Green Version]
- Tikhanovich, I.; Cox, J.; Weinman, S.A. Forkhead box class O transcription factors in liver function and disease. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 1), 125–131. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.C.; Copps, K.D.; Guo, S.; Li, Y.; Kollipara, R.; DePinho, R.A.; White, M.F. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell. Metab. 2008, 8, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Wan, M.; Leavens, K.F.; Chu, Q.; Monks, B.R.; Fernandez, S.; Ahima, R.S.; Ueki, K.; Kahn, C.R.; Birnbaum, M.J. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 2012, 18, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Park, J.E.; Lee, M.; Hardwick, J.P. Hepatic lipid homeostasis by peroxisome proliferator-activated receptor gamma 2. Liver Res. 2018, 2, 209–215. [Google Scholar] [CrossRef]
- Inoue, M.; Ohtake, T.; Motomura, W.; Takahashi, N.; Hosoki, Y.; Miyoshi, S.; Suzuki, Y.; Saito, H.; Kohgo, Y.; Okumura, T. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem. Biophys. Res. Commun. 2005, 336, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motomura, W.; Inoue, M.; Ohtake, T.; Takahashi, N.; Nagamine, M.; Tanno, S.; Kohgo, Y.; Okumura, T. Up-regulation of ADRP in fatty liver in human and liver steatosis in mice fed with high fat diet. Biochem. Biophys. Res. Commun. 2006, 340, 1111–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, H.; Kiyono, S.; Kondo, T.; Sekimoto, T.; Yokosuka, O. Palmitate-induced Regulation of PPARγ via PGC1α: A Mechanism for Lipid Accumulation in the Liver in Nonalcoholic Fatty Liver Disease. Int. J. Med. Sci. 2016, 13, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gu, M.; Cai, W.; Yu, L.; Feng, L.; Zhang, L.; Zang, Q.; Wang, Y.; Wang, D.; Chen, H.; et al. Dietary component isorhamnetin is a PPARγ antagonist and ameliorates metabolic disorders induced by diet or leptin deficiency. Sci. Rep. 2016, 6, 19288. [Google Scholar] [CrossRef]
- Knouff, C.; Auwerx, J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: Lessons from genetics and pharmacology. Endocr. Rev. 2004, 25, 899–918. [Google Scholar] [CrossRef] [Green Version]
- Cock, T.A.; Houten, S.M.; Auwerx, J. Peroxisome proliferator-activated receptor-gamma: Too much of a good thing causes harm. EMBO Rep. 2004, 5, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bing, C. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte 2015, 4, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Hong, P.; Gu, R.N.; Li, F.X.; Xiong, X.X.; Liang, W.B.; You, Z.J.; Zhang, H.F. NLRP3 inflammasome as a potential treatment in ischemic stroke concomitant with diabetes. J. Neuroinflamm. 2019, 16, 121. [Google Scholar] [CrossRef]
- Rider, P.; Carmi, Y.; Guttman, O.; Braiman, A.; Cohen, I.; Voronov, E.; White, M.R.; Dinarello, C.A.; Apte, R.N. IL-1α and IL-1β Recruit Different Myeloid Cells and Promote Different Stages of Sterile Inflammation. J. Immunol. 2011, 187, 4835–4843. [Google Scholar] [CrossRef] [Green Version]
- Nov, O.; Shapiro, H.; Ovadia, H.; Tarnovscki, T.; Dvir, I.; Shemesh, E.; Kovsan, J.; Shelef, I.; Carmi, Y.; Voronov, E.; et al. Interleukin-1β regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS ONE 2013, 8, e53626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fukumoto, H.; Koh, G.; Yano, H.; Yasuda, K.; Masuda, K.; Ikeda, H.; Imura, H.; Seino, Y. Liver and muscle-fat type glucose transporter gene expression in obese and diabetic rats. Biochem. Biophys. Res. Commun. 1991, 175, 995–1002. [Google Scholar] [CrossRef]
- Cao, L.; Mao, C.; Li, S.; Zhang, Y.; Lv, J.; Jiang, S.; Xu, Z. Hepatic Insulin Signaling Changes: Possible Mechanism in Prenatal Hypoxia-Increased Susceptibility of Fatty Liver in Adulthood. Endocrinology 2012, 153, 4955–4965. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kwon, M.-S.; Son, J.; Kang, S.-W.; Song, Y. Selective effect of phosphatidylcholine on the lysis of adipocytes. PLoS ONE 2017, 12, e0176722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreurs, M.; Kuipers, F.; Van Der Leij, F.R. Regulatory enzymes of mitochondrial β-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010, 11, 380–388. [Google Scholar] [CrossRef]
- Keung, W.; Ussher, J.R.; Jaswal, J.S.; Raubenheimer, M.; Lam, V.H.M.; Wagg, C.S.; Lopaschuk, G.D. Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes 2013, 62, 711–720. [Google Scholar] [CrossRef] [Green Version]
- Ratheiser, K.; Schneeweiss, B.; Waldhäusl, W.; Fasching, P.; Korn, A.; Nowotny, P.; Rohac, M.; Wolf, H.P. Inhibition by etomoxir of carnitine palmitoyltransferase I reduces hepatic glucose production and plasma lipids in non-insulin-dependent diabetes mellitus. Metabolism 1991, 40, 1185–1190. [Google Scholar] [CrossRef]
- Luijten, I.H.N.; Feldmann, H.M.; Essen, G.V.; Cannon, B.; Nedergaard, J. In the absence of UCP1-mediated diet-induced thermogenesis, obesity is augmented even in the obesity-resistant 129S mouse strain. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E729–E740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Liu, X.; Kris-Etherton, P.M.; West, S.G.; Lamarche, B.; Jenkins, D.J.A.; Fleming, J.A.; McCrea, C.E.; Pu, S.; Couture, P.; Connelly, P.W.; et al. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obesity 2016, 24, 2261–2268. [Google Scholar] [CrossRef] [Green Version]
- Elsawy, G.; Abdelrahman, O.; Hamza, A. Effect of Choline Supplementation on Rapid Weight Loss and Biochemical Variables Among Female Taekwondo and Judo Athletes. J. Hum. Kinet. 2014, 40, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Noh, Y.; Heo, C.-Y. The effect of phosphatidylcholine and deoxycholate compound injections to the localized adipose tissue: An experimental study with a murine model. Arch. Plast. Surg. 2012, 39, 452–456. [Google Scholar] [CrossRef] [Green Version]
- Gundermann, K.J.; Hasengschwandtner, F.; Lettko, M. The role of polyenylphosphatidylcholine (PPC) for the reduction of fat pads in deoxycholate containing injectables. Med. Res. Arch. 2017, 5. Available online: https://journals.ke-i.org/index.php/mra/article/view/1441 (accessed on 14 October 2020).
- Duncan, D.I.; Hasengschwandtner, F. Lipodissolve for Subcutaneous Fat Reduction and Skin Retraction. Aesthetic Surg. J. 2005, 25, 530–543. [Google Scholar] [CrossRef] [Green Version]
- Rose, P.T.; Morgan, M. Histological changes associated with mesotherapy for fat dissolution. J. Cosmet. Laser. 2005, 7, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D.; Zilberman, Y.; Greenzaid, P.; Zamir, S. Structural and kinetic studies on the solubilization of lecithin by sodium deoxycholate. Biochemistry 1979, 18, 3517–3525. [Google Scholar] [CrossRef]
- Chen, H.; Simar, D.; Morris, M.J. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: Interaction with postnatal nutritional environment. PLoS ONE 2009, 4, e6259. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Chen, H.; Simar, D.; Pegg, K.; Saad, S.; Palmer, C.; Morris, M.J. Exendin-4 is effective against metabolic disorders induced by intrauterine and postnatal overnutrition in rodents. Diabetologia 2014, 57, 614–622. [Google Scholar] [CrossRef]
- Chan, Y.L.; Saad, S.; Al-Odat, I.; Oliver, B.G.; Pollock, C.; Jones, N.M.; Chen, H. Maternal L-Carnitine Supplementation Improves Brain Health in Offspring from Cigarette Smoke Exposed Mothers. Front. Mol. Neurosci. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Soga, Y.; Iwamoto, Y.; Yoshizawa, S.; Iwata, H.; Kokeguchi, S.; Takashiba, S.; Nishimura, F. Macrophage-Adipocyte Interaction: Marked Interleukin-6 Production by Lipopolysaccharide. Obesity 2007, 15, 2549–2552. [Google Scholar] [CrossRef] [PubMed]











| Parameters | Chow-PBS | Chow-UTS-001 (0.6 mg) | Chow-UTS-001 (6 mg) | HFD-PBS | HFD-UTS-001 (0.6 mg) | HFD-UTS-001 (6 mg) |
|---|---|---|---|---|---|---|
| Body weight at 0 week (g) | 19.9 ± 0.3 | 20.3 ± 0.1 | 19.8 ± 0.3 | 19.5 ± 0.3 | 20.1 ± 0.3 | 19.9 ± 0.3 |
| Body weight at 10 weeks (g) | 28.7 ± 0.5 | 29.5 ± 0.5 | 28.1 ± 0.4 | 37.1 ± 0.8 **** | 38.3 ± 1.5 **** | 33.8 ± 1.2 ****,## |
| Twenty-four calorie intake (kJ/mouse/day) | 36.18 ± 2.18 | 28.05 ± 0.69 | 37.45 ± 3.25 | 238.4 ± 33.13 **** | 318.53 ± 37.27 ****# | 261.41 ± 30.06 **** |
| Kidney (g) | 0.192 ± 0.005 | 0.193 ± 0.012 | 0.181 ± 0.009 | 0.215 ± 0.007 * | 0.213 ± 0.014 | 0.213 ± 0.009 |
| % | 0.690 ± 0.018 | 0.690 ± 0.018 | 0.670 ± 0.031 | 0.603 ± 0.027 * | 0.592 ± 0.021 * | 0.642 ± 0.019 |
| Liver (g) | 1.18 ± 0.005 | 1.27 ± 0.03 | 1.22 ± 0.04 | 1.56 ± 0.07 **** | 1.58 ± 0.07 ** | 1.400 ± 0.040 **,# |
| % | 4.23 ± 0.15 | 0.683 ± 0.037 | 4.51 ± 0.12 | 4.30 ± 0.12 | 4.46 ± 0.31 | 4.23 ± 0.11 |
| Heart (g) | 0.143 ± 0.005 | 0.146 ± 0.007 | 0.139 ± 0.008 | 0.159 ± 0.006 | 0.150 ± 0.008 | 0.149 ± 0.012 |
| % | 0.511 ± 0.012 | 0.514 ± 0.018 | 0.517 ± 0.030 | 0.433 ± 0.015 * | 0.422 ± 0.019 ** | 0.433 ± 0.018 * |
| Spleen (g) | 0.096 ± 0.012 | 0.087 ± 0.007 | 0.085 ± 0.014 | 0.110 ± 0.007 | 0.096 ± 0.015 | 0.105 ± 0.011 |
| % | 0.343 ± 0.041 | 0.308 ± 0.021 | 0.312 ± 0.042 | 0.298 ± 0.014 | 0.259 ± 0.029 | 0.305 ± 0.020 |
| Rp fat (g) | 0.091 ± 0.010 | 0.107 ± 0.012 | 0.077 ± 0.013 | 0.680 ± 0.057 **** | 0.767 ± 0.111 **** | 0.405 ± 0.070 ***,## |
| % | 0.324 ± 0.033 | 0.380 ± 0.040 | 0.284 ± 0.046 | 1.86 ± 0.12 **** | 2.17 ± 0.36 **** | 1.19 ± 0.18 ****,## |
| Testicular fat (g) | 0.381 ± 0.017 | 0.444 ± 0.044 | 0.312 ± 0.034 | 1.81 ± 0.13 **** | 1.93 ± 0.15 **** | 1.04 ± 0.16 ****,#### |
| % | 1.37 ± 0.06 | 1.57 ± 0.15 | 1.16 ± 0.12 | 4.95 ± 0.29 **** | 5.36 ± 0.30 **** | 3.05 ± 0.40 ****,#### |
| Parameters | Chow-PBS | Chow-UTS-001 (0.6 mg) | Chow-UTS-001 (6 mg) | HFD-PBS | HFD-UTS-001 (0.6 mg) | HFD-UTS-001 (6 mg) |
|---|---|---|---|---|---|---|
| Serum TG (mg/mL) | 0.94 ± 0.032 | 1.15 ± 0.05 | 1.06 ± 0.047 | 1.12 ± 0.047* | 1.21 ± 0.062 ** | 1.19 ± 0.062 *** |
| Serum FFA (nM) | 4.82 ± 0.21 | 4.74 ± 0.27 | 4.9 ± 0.25 | 5.49 ± 0.35 | 5.95 ± 0.48 | 4.8 ± 0.15 |
| Liver Oil Red O staining (OD/mg of tissue) | 0.0057 ± 0.00032 | 0.0066 ± 0.00059 | 0.00501 ± 0.00031 | 0.01898 ± 0.00321 *** | 0.02489 ± 0.0016 **** | 0.019 ± 0.00346 **** |
| Glucose (mM) | 12.8 ± 1.2 | 12.9 ± 1.4 | 14.8 ± 0.3 | 17.5 ± 1.2 ** | 19.15 ± 0.9 *** | 18.1 ± 1.1 ** |
| Serum insulin (ng/mL) | 0.399 ± 0.02 | 0.40 ± 0.04 | 0.409 ± 0.04 | 0.412 ± 0.02 | 0.399 ± 0.03 | 0.415 ± 0.03 |
| HOMA-IR | 0.22 ± 0.02 | 0.24 ± 0.03 | 0.27 ± 0.01 | 0.34 ± 0.02 **** | 0.34 ± 0.01 *** | 0.32 ± 0.02 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komalla, V.; Sheikholeslami, B.; Li, G.; Bokshi, B.; Chan, Y.L.; Ung, A.; Gregory Oliver, B.; Chen, H.; Haghi, M. Impact of A Cargo-Less Liposomal Formulation on Dietary Obesity-Related Metabolic Disorders in Mice. Int. J. Mol. Sci. 2020, 21, 7640. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207640
Komalla V, Sheikholeslami B, Li G, Bokshi B, Chan YL, Ung A, Gregory Oliver B, Chen H, Haghi M. Impact of A Cargo-Less Liposomal Formulation on Dietary Obesity-Related Metabolic Disorders in Mice. International Journal of Molecular Sciences. 2020; 21(20):7640. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207640
Chicago/Turabian StyleKomalla, Varsha, Behjat Sheikholeslami, Gerard Li, Bishwajit Bokshi, Yik Lung Chan, Alison Ung, Brian Gregory Oliver, Hui Chen, and Mehra Haghi. 2020. "Impact of A Cargo-Less Liposomal Formulation on Dietary Obesity-Related Metabolic Disorders in Mice" International Journal of Molecular Sciences 21, no. 20: 7640. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207640