Induction of a Specific Humoral Immune Response by Nasal Delivery of Bcla2ctd of Clostridioides difficile
Abstract
:1. Introduction
2. Results
2.1. Purified Bcla2ctd Has Low Stability
2.2. Bcla2ctd Is Efficiently Displayed on B. Subtilis Spores
2.3. Bcla2ctd Is Stabilized by the Adsorption on B. Subtilis Spores
2.4. Adsorption of Bcla2ctd Increases Spore Adherence to Caco-2 Cells
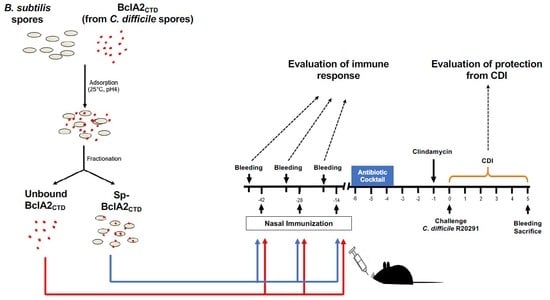
2.5. Intranasal Immunization
2.6. Effect of Nasal- Bcla2ctd Immunization Against C. Difficile R20291 Infection
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Spore Purification
4.2. BclA2CTD over-Production and Purification
4.3. Adsorption Reaction, Stability, and Production of Spores for Animal Immunization
4.4. Western and Dot-Blot Analyses
4.5. Adherence to Caco-2 Cells
4.6. Animals
4.7. Immunization Regimen in Mice
4.8. Animal Infection Model
4.9. Quantification of Spores from Feces and Colon Samples
4.10. Evaluation of Bcla2ctd-Specific Igg Levels in Mice Serum
4.11. Immunofluorescence Analysis
4.12. Cytotoxicity Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CDI | Clostridioides difficile infection |
| BclA | Bacillus collagen-like protein of anthracis |
| NTD | N-terminal domain |
| CTD | C-terminal domain |
| BclA2CTD | C-terminal domain of BclA2 |
| ST | Standard |
| PBS | Phosphate-buffered saline |
| Sp | Spores of B. subtilis |
| Sp-BclA2CTD | Spores of B. subtilis adsorbed with BclA2CTD |
| ANOVA | Analysis of variance |
| ELISA | Enzyme-linked immunosorbent assay |
| IgG | Immunoglobulin G |
| I.P | Intraperitoneal |
| Wt | Wild-type |
| PI | Pre-Immune |
| d13 | Day 13 |
| d27 | Day 27 |
| OD | Optical density |
| Fl.I | Fluorescence intensity |
| CFU | Colony forming unit |
References
- Kelly, C.P.; Pothoulakis, C.; LaMont, J.T. Clostridium difficile colitis. N. Engl. J. Med. 1994, 330, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Heinlen, L.; Ballard, J.D. Clostridium difficile infection. Am. J. Med. Sci. 2010, 340, 247–252. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Killgore, G.E.; Thompson, A.; Owens, R.C., Jr.; Kazakova, S.V.; Sambol, S.P.; Johnson, S.; Gerding, D.N. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 2005, 353, 2433–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warny, M.; Pepin, J.; Fang, A.; Killgore, G.; Thompson, A.; Brazier, J.; Frost, E.; McDonald, L.C. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005, 366, 1079–1084. [Google Scholar] [CrossRef]
- Hookman, P.; Barkin, J.S. Clostridium difficile associated infection, diarrhea and colitis. World J. Gastroenterol. 2009, 15, 1554–1580. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A. Current and emerging management options for Clostridium difficile infection: What is the role of fidaxomicin? Clin. Microbiol. Infect. 2012, 18 (Suppl. 6), 28–35. [Google Scholar] [CrossRef] [Green Version]
- Valiente, E.; Dawson, L.F.; Cairns, M.D.; Stabler, R.A.; Wren, B.W. Emergence of new PCR ribotypes from the hypervirulent Clostridium difficile 027 lineage. J. Med. Microbiol. 2012, 61, 49–56. [Google Scholar] [CrossRef]
- McFarland, L.V.; Elmer, G.W.; Surawicz, C.M. Breaking the cycle: Treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am. J. Gastroenterol. 2002, 97, 1769–1775. [Google Scholar] [CrossRef]
- Feher, C.; Soriano, A.; Mensa, J. A review of experimental and off-label therapies for Clostridium difficile infection. Infect. Dis. 2017, 6, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, E.J.; Bhalodia, S.; Jacob, S.; Patel, H.; Trinh, K.V.; Varghese, B.; Yang, J.; Young, S.R.; Raffa, R.B. Clostridium difficile infection: A brief update on emerging therapies. Am. J. Health. Syst. Pharm. 2015, 72, 1007–1012. [Google Scholar] [CrossRef]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.Y.; Dirweesh, A.; Khurshid, T.; Siddiqui, W.J. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Maziade, P.J.; Pereira, P.; Goldstein, E.J. A Decade of Experience in Primary Prevention of Clostridium difficile Infection at a Community Hospital Using the Probiotic Combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin. Infect. Dis. 2015, 60 (Suppl. 2), S144–S147. [Google Scholar] [CrossRef]
- Diaz-Gonzalez, F.; Milano, M.; Olguin-Araneda, V.; Pizarro-Cerda, J.; Castro-Cordova, P.; Tzeng, S.C.; Maier, C.S.; Sarker, M.R.; Paredes-Sabja, D. Protein composition of the outermost exosporium-like layer of Clostridium difficile 630 spores. J. Proteom. 2015, 123, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mora-Uribe, P.; Miranda-Cardenas, C.; Castro-Cordova, P.; Gil, F.; Calderon, I.; Fuentes, J.A.; Rodas, P.I.; Banawas, S.; Sarker, M.R.; Paredes-Sabja, D. Characterization of the Adherence of Clostridium difficile Spores: The Integrity of the Outermost Layer Affects Adherence Properties of Spores of the Epidemic Strain R20291 to Components of the Intestinal Mucosa. Front. Cell. Infect. Microbiol. 2016, 6, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizarro-Guajardo, M.; Olguin-Araneda, V.; Barra-Carrasco, J.; Brito-Silva, C.; Sarker, M.R.; Paredes-Sabja, D. Characterization of the collagen-like exosporium protein, BclA1, of Clostridium difficile spores. Anaerobe 2014, 25, 18–30. [Google Scholar] [CrossRef]
- Ricca, E.; Baccigalupi, L.; Cangiano, G.; De Felice, M.; Isticato, R. Mucosal vaccine delivery by non-recombinant spores of Bacillus subtilis. Microb. Cell. Fact. 2014, 13, 115. [Google Scholar] [CrossRef] [Green Version]
- Isticato, R.; Ricca, E. Spore Surface Display. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. Febs. Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Sidney, J.; Dow, C.; Mothe, B.; Sette, A.; Peters, B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLOS. Comput. Biol. 2008, 4, e1000048. [Google Scholar] [CrossRef] [Green Version]
- Moutaftsi, M.; Peters, B.; Pasquetto, V.; Tscharke, D.C.; Sidney, J.; Bui, H.H.; Grey, H.; Sette, A. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat. Biotechnol. 2006, 24, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; Hong, H.A.; Van Tong, H.; Hoang, T.H.; Brisson, A.; Cutting, S.M. Mucosal delivery of antigens using adsorption to bacterial spores. Vaccine 2010, 28, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Sirec, T.; Treppiccione, L.; Maurano, F.; De Felice, M.; Rossi, M.; Ricca, E. Non-recombinant display of the B subunit of the heat labile toxin of Escherichia coli on wild type and mutant spores of Bacillus subtilis. Microb. Cell. Fact. 2013, 12, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngman, P.; Perkins, J.B.; Losick, R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid 1984, 12, 1–9. [Google Scholar] [CrossRef]
- Isticato, R.; Ricca, E.; Baccigalupi, L. Spore Adsorption as a Nonrecombinant Display System for Enzymes and Antigens. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Barkin, J.A.; Sussman, D.A.; Fifadara, N.; Barkin, J.S. Clostridium difficile Infection and Patient-Specific Antimicrobial Resistance Testing Reveals a High Metronidazole Resistance Rate. Dig. Dis. Sci. 2017, 62, 1035–1042. [Google Scholar] [CrossRef]
- Johnson, S.; Sanchez, J.L.; Gerding, D.N. Metronidazole resistance in Clostridium difficile. Clin. Infect. Dis. 2000, 31, 625–626. [Google Scholar] [CrossRef]
- Potocki, W.; Negri, A.; Peszynska-Sularz, G.; Hinc, K.; Obuchowski, M.; Iwanicki, A. The combination of recombinant and non-recombinant Bacillus subtilis spore display technology for presentation of antigen and adjuvant on single spore. Microb. Cell. Fact. 2017, 16, 151. [Google Scholar] [CrossRef] [Green Version]
- Permpoonpattana, P.; Hong, H.A.; Phetcharaburanin, J.; Huang, J.M.; Cook, J.; Fairweather, N.F.; Cutting, S.M. Immunization with Bacillus spores expressing toxin A peptide repeats protects against infection with Clostridium difficile strains producing toxins A and B. Infect. Immun. 2011, 79, 2295–2302. [Google Scholar] [CrossRef] [Green Version]
- Ghose, C.; Eugenis, I.; Edwards, A.N.; Sun, X.; McBride, S.M.; Ho, D.D. Immunogenicity and protective efficacy of Clostridium difficile spore proteins. Anaerobe 2016, 37, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Jang, Y.S. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin. Exp. Vaccine. Res. 2017, 6, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sirec, T.; Strazzulli, A.; Isticato, R.; De Felice, M.; Moracci, M.; Ricca, E. Adsorption of beta-galactosidase of Alicyclobacillus acidocaldarius on wild type and mutants spores of Bacillus subtilis. Microb. Cell. Fact. 2012, 11, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Hong, H.A.; Huang, J.M.; Colenutt, C.; Khang, D.D.; Nguyen, T.V.; Park, S.M.; Shim, B.S.; Song, H.H.; Cheon, I.S.; et al. Killed Bacillus subtilis spores as a mucosal adjuvant for an H5N1 vaccine. Vaccine 2012, 30, 3266–3277. [Google Scholar] [CrossRef] [PubMed]
- Reljic, R.; Sibley, L.; Huang, J.M.; Pepponi, I.; Hoppe, A.; Hong, H.A.; Cutting, S.M. Mucosal vaccination against tuberculosis using inert bioparticles. Infect. Immun. 2013, 81, 4071–4080. [Google Scholar] [CrossRef] [Green Version]
- Harwood, C.R.; Cutting, S.M. Molecular Biological Methods for Bacillus; Wiley: Chichester, NY, USA, 1990; p. xxxv. 581p. [Google Scholar]
- Calderon-Romero, P.; Castro-Cordova, P.; Reyes-Ramirez, R.; Milano-Cespedes, M.; Guerrero-Araya, E.; Pizarro-Guajardo, M.; Olguin-Araneda, V.; Gil, F.; Paredes-Sabja, D. Clostridium difficile exosporium cysteine-rich proteins are essential for the morphogenesis of the exosporium layer, spore resistance, and affect C. difficile pathogenesis. Plos. Pathog. 2018, 14, e1007199. [Google Scholar] [CrossRef]
- Edwards, A.N.; McBride, S.M. Isolating and Purifying Clostridium difficile Spores. Methods. Mol. Biol. 2016, 1476, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Isticato, R.; Pelosi, A.; De Felice, M.; Ricca, E. CotE binds to CotC and CotU and mediates their interaction during spore coat formation in Bacillus. subtilis. J. Bacteriol. 2010, 192, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Paredes-Sabja, D.; Sarker, M.R. Adherence of Clostridium difficile spores to Caco-2 cells in culture. J. Med. Microbiol. 2012, 61, 1208–1218. [Google Scholar] [CrossRef]
- Chantret, I.; Rodolosse, A.; Barbat, A.; Dussaulx, E.; Brot-Laroche, E.; Zweibaum, A.; Rousset, M. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: Evidence for glucose-dependent negative regulation. J. Cell. Sci. 1994, 107 Pt 1, 213–225. [Google Scholar]
- Chen, X.; Katchar, K.; Goldsmith, J.D.; Nanthakumar, N.; Cheknis, A.; Gerding, D.N.; Kelly, C.P. A mouse model of Clostridium difficile-associated disease. Gastroenterology 2008, 135, 1984–1992. [Google Scholar] [CrossRef]
- Warren, C.A.; van Opstal, E.J.; Riggins, M.S.; Li, Y.; Moore, J.H.; Kolling, G.L.; Guerrant, R.L.; Hoffman, P.S. Vancomycin treatment’s association with delayed intestinal tissue injury, clostridial overgrowth, and recurrence of Clostridium difficile infection in mice. Antimicrob. Agents. Chemother. 2013, 57, 689–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deakin, L.J.; Clare, S.; Fagan, R.P.; Dawson, L.F.; Pickard, D.J.; West, M.R.; Wren, B.W.; Fairweather, N.F.; Dougan, G.; Lawley, T.D. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun. 2012, 80, 2704–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theriot, C.M.; Koumpouras, C.C.; Carlson, P.E.; Bergin, I.I.; Aronoff, D.M.; Young, V.B. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut. Microbes. 2011, 2, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, A.R.; Reyes-Ramírez, R.; Pizarro-Guajardo, M.; Saggese, A.; Castro-Córdova, P.; Isticato, R.; Ricca, E.; Paredes-Sabja, D.; Baccigalupi, L. Induction of a Specific Humoral Immune Response by Nasal Delivery of Bcla2ctd of Clostridioides difficile. Int. J. Mol. Sci. 2020, 21, 1277. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041277
Maia AR, Reyes-Ramírez R, Pizarro-Guajardo M, Saggese A, Castro-Córdova P, Isticato R, Ricca E, Paredes-Sabja D, Baccigalupi L. Induction of a Specific Humoral Immune Response by Nasal Delivery of Bcla2ctd of Clostridioides difficile. International Journal of Molecular Sciences. 2020; 21(4):1277. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041277
Chicago/Turabian StyleMaia, Ana Raquel, Rodrigo Reyes-Ramírez, Marjorie Pizarro-Guajardo, Anella Saggese, Pablo Castro-Córdova, Rachele Isticato, Ezio Ricca, Daniel Paredes-Sabja, and Loredana Baccigalupi. 2020. "Induction of a Specific Humoral Immune Response by Nasal Delivery of Bcla2ctd of Clostridioides difficile" International Journal of Molecular Sciences 21, no. 4: 1277. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041277